Total synthesis of spiromastilactone A†
Abstract
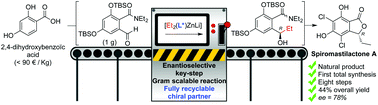
The total synthesis of spiromastilactone A is reported for the first time. A swift strategy is presented that involves a pivotal enantioselective nucleophilic 1,2-alkylation of an aldehyde prepared in four quantitative synthetic steps from commercial 2,4-dihydroxybenzoic acid. This key reaction, which was described very recently by our group and carried out here on a gram scale, involves cheap and easily accessible tricoordinated chiral lithium amido zincates. The resulting enantioenriched secondary alcohol is involved afterward in an efficient intramolecular cyclization providing the phthalide core of the target, and two quantitative additional steps aiming to deprotect the phenol groups then introduce chlorine atoms end the synthetic scheme. Spiromastilactone A is obtained in 44% overall yield in eight synthetic steps, among which six are quantitative, and the 89 : 11 enantiomeric ratio (78% ee value) is in favor of the right enantiomer (R configuration).
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...