Open Access Article
Open Access ArticleAchiral amino acid glycine acts as an origin of homochirality in asymmetric autocatalysis†
Arimasa
Matsumoto‡
 a,
Hanae
Ozaki
a,
Sumeru
Tsuchiya
a,
Toru
Asahi
b,
Meir
Lahav
c,
Tsuneomi
Kawasaki
a,
Hanae
Ozaki
a,
Sumeru
Tsuchiya
a,
Toru
Asahi
b,
Meir
Lahav
c,
Tsuneomi
Kawasaki
 a and
Kenso
Soai
a and
Kenso
Soai
 *a
*a
aDepartment of Applied Chemistry, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: soai@rs.kagu.tus.ac.jp
bDepartment of Advanced Science and Engineering, Graduate School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku-ku, Tokyo 169-8555, Japan
cWeizmann Institute of Science, Rehovot, Israel
First published on 1st April 2019
Abstract
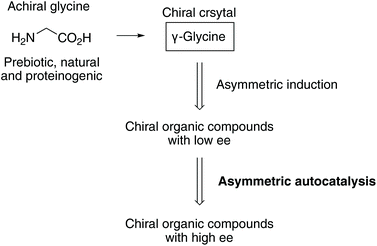
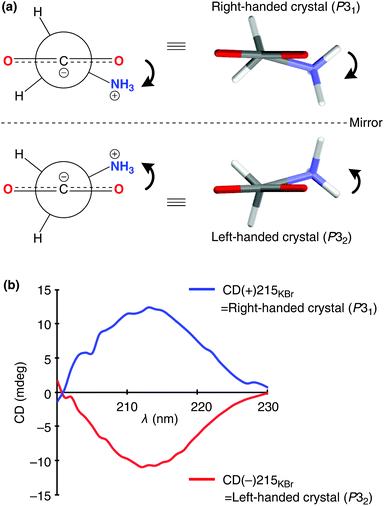
Chiral crystals of the only achiral proteinogenic α-amino acid, glycine induced the asymmetric autocatalysis with amplification of enantiomeric excess (ee). The P31 crystals of γ-glycine, which display positive Cotton effect (CD) at around 215 nm, mediate the asymmetric autocatalysis to yield (R)-pyrimidyl alkanol with high ee. In contrast, the enantiomorphic P32 crystals, which display negative Cotton effect, afford (S)-alkanol after the significant amplification of ee by asymmetric autocatalysis.
The origin of biological homochirality such as L-amino acids and D-sugars has been a long-standing subject of considerable interest.1–16 Among the 20 natural amino acids, the only achiral glycine,17–19 with no asymmetric carbon atom, stands as the simplest (Fig. S1, ESI†). Indeed, when the chirality of natural L-amino acids is mentioned, we often neglect the existence of achiral glycine. Therefore, except for the enantiomer-selective occlusion of the other amino acids,20,21 it is believed that achiral glycine does not possess any role in the origin of chirality of organic compounds.
Nevertheless, the stable γ-glycine polymorph, which belongs to the Sohncke space group (P31 or P32), is known to have a chiral crystalline structure (Scheme 1).22 However, a long-standing problem has been that the absolute crystal structure of γ-glycine could not be elucidated. We recently determined the absolute chiral crystalline structure of γ-glycine by single-crystal X-ray crystallography and optical rotatory dispersion.23 Furthermore, the CD spectra of crystalline chiral γ-glycine was reported very recently by Guillemin.24
Asymmetric autocatalysis is a reaction in which a chiral product acts as a chiral catalyst for its own production.25–42 We have been studying asymmetric autocatalysis of pyrimidyl alkanol in the enantioselective addition of diisopropylzinc (i-Pr2Zn) to pyrimidine-5-carbaldehydes. The isopropylzinc alkoxide of 5-pyrimidyl alkanol43–50 acts as a highly efficient asymmetric autocatalyst with amplification of enantiomeric excess (ee), wherein a very small enantiomeric imbalance can be significantly increased, leading to a nearly enantiopure state (>99.5% ee) during the consecutive reactions. Therefore, chiral factors51–57 can act as a source of chirality to trigger the asymmetric autocatalytic amplification of ee.
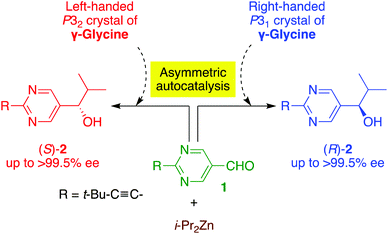
Here we report the following findings: (1) the correlation between the absolute crystal structure of γ-glycine and the CD spectra, and (2) the chiral γ-polymorph of glycine acts as a chiral trigger of asymmetric autocatalysis to produce pyrimidyl alkanol with the absolute configuration corresponding to that of the γ-glycine crystal (Schemes 1 and 2).
The absolute structure of each enantiomorph of the γ-glycine has been determined by anomalous X-ray diffraction. The CD spectrum of each enantiomorph was determined by solid-state circular dichroism (CD) analysis in a KBr matrix (Table S1, ESI†). In the crystal lattice, glycine molecules assume a twisted chiral configuration. The dihedral angle between the plane of the carboxylate and that containing the C–C–N atoms is ca. 15.4°. It has been confirmed that the glycine molecules in the crystal of space group P31, which are in right-handed twisted configuration, induce a positive Cotton effect at around 215 nm (Fig. 1); in contrast, crystals formed from glycine with left-handed configuration (space group: P32) induce a negative Cotton effect.
The enantiomorphic γ-glycine was then used as a heterogeneous chiral initiator for asymmetric autocatalysis of 5-pyrimidyl alkanol 2 (Scheme 2 and Table 1). In a typical procedure for asymmetric autocatalysis, an enantiomorphic crystal of γ-glycine was ground into a fine powder together with aldehyde 1. To this powder, toluene solution of i-Pr2Zn was slowly added and reacted for 12 h. Then, toluene solutions of i-Pr2Zn and aldehyde 1 were slowly added. After finishing the reaction, the ee was determined by analysing purified 5-pyrimidyl alkanol 2 by chiral HPLC (for the experimental details, see ESI†).
| Entrya | γ-Glycine | 5-Pyrimidyl alkanol 2 | |||
|---|---|---|---|---|---|
| CDb | Helicity | Yieldc (%) | eed (%) | Config. | |
a The molar ratio of γ-glycine/1/i-Pr2Zn was 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 (mmol).
b The Cotton effect of solid-state CD (KBr disk) at around 215 nm was indicated.
c The yield of isolated product 2.
d The ee value was determined by HPLC on a chiral stationary phase.
e Ee can be amplified by further asymmetric autocatalysis. See footnote f.
f After the typical experimental method, additional rounds of consecutive asymmetric autocatalysis were performed after the isolation of 2.27 0.7 (mmol).
b The Cotton effect of solid-state CD (KBr disk) at around 215 nm was indicated.
c The yield of isolated product 2.
d The ee value was determined by HPLC on a chiral stationary phase.
e Ee can be amplified by further asymmetric autocatalysis. See footnote f.
f After the typical experimental method, additional rounds of consecutive asymmetric autocatalysis were performed after the isolation of 2.27
|
|||||
| 1 | (+) | Right | 68 | 73 | R |
| 2 | (−) | Left | 52 | 69 | S |
| 3 | (+) | Right | 59 | 43 | R |
| 4 | (−) | Left | 66 | 63 | S |
| 5 | (+) | Right | 69 | 35e | R |
| 6 | (−) | Left | 66 | 52 | S |
| 7 | (+) | Right | 67 (94)f | 57 (>99.5)f | R |
| 8 | (−) | Left | 67 (89)f | 72 (>99.5)f | S |
As shown in entry 1, when pyrimidine-5-carbaldehyde 1 and i-Pr2Zn reacted in the presence of P31 crystals, enantioenriched (R)-pyrimidyl alkanol 2 was obtained with 73% ee after the subsequent asymmetric autocatalysis, through addition of aldehyde 1 and i-Pr2Zn. On the other hand, P32 γ-glycine induced the production of enantioenriched (S)-alkanol 2 with 69% ee (entry 2). The reproducibility of the stereochemical relationships between the crystal chirality of γ-glycine and the resulting alkanol 2 is shown in entries 3–6 of Table 1. It should be noted that additional consecutive asymmetric autocatalysis afforded almost enantiopure (R)- or (S)-2 with >99.5% ee (entries 7 and 8). Therefore, it was found that the crystalline chirality of the achiral glycine is responsible for the enantioselective addition of i-Pr2Zn to aldehyde 1; thus, the crystalline chirality, which comes from the solid-state chiral conformation of glycine molecules (P31 or P32), induces a tiny enantiomeric imbalance during the formation of the zinc alkoxide in the initial stage of the reaction. During the subsequent asymmetric autocatalysis, this tiny enantiomeric imbalance is significantly amplified to afford the near enantiopure alkanol 2 with the absolute configuration corresponding to handedness of γ-glycine.
This sequence of reactions represents one of the chemical processes in which the scenario for the evolution of enantioenriched chiral organic compounds from the achiral natural amino acid glycine was achieved in real chemical reactions (Scheme 1). The process of asymmetric induction in the organic product with an asymmetric carbon atom and the amplification of chirality through asymmetric autocatalysis indicate the possibility of the simplest α-amino acid glycine being the origin of chirality.
The initial enantioimbalance of asymmetric autocatalyst 2 is likely generated on the chiral surface of γ-glycine,58 because the dissolution of the crystal causes the disappearance of chirality. We postulated the possible mechanisms of asymmetric induction59 are as follows: (1) enantioface-selective adsorption of aldehyde 1 followed by i-Pr2Zn addition, and (2) enantioselective adsorption of the initially formed alkanol 2 (as isopropylzinc alkoxide), which can induce an enantiomerically imbalanced autocatalyst. Subsequent asymmetric autocatalysis then enhances the small ee of the asymmetric autocatalyst to a nearly enantiopure state.
In conclusion, we have established the correlation between the absolute crystal structure of γ-glycine and its CD spectra; we found that chiral γ-glycine crystal acts as a chiral trigger for asymmetric autocatalysis to afford a highly enantioenriched organic compound. The implication of the present results for the origin of homochirality is that achiral glycine, which has long been neglected as irrelevant to chirality, may play an important role as a chiral γ-glycine crystal as a chiral trigger for asymmetric autocatalysis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science and MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016.Notes and references
- K. Mislow, Collect. Czech. Chem. Commun., 2003, 68, 849 CrossRef CAS
.
- M. Bolli, R. Micura and A. Eschenmoser, Chem. Biol., 1997, 4, 309 CrossRef CAS
.
- D. K. Kondepudi, R. Kaufman and N. Singh, Science, 1990, 250, 975 CrossRef CAS PubMed
.
- J. M. Ribo, J. Crusats, F. Sagues, J. Claret and R. Rubires, Science, 2001, 292, 2063 CrossRef CAS PubMed
.
- B. L. Feringa and R. A. van Delden, Angew. Chem., Int. Ed., 1999, 38, 3418 CrossRef
.
- I. Weissbuch and M. Lahav, Chem. Rev., 2011, 111, 3236 CrossRef CAS
.
- K.-H. Ernst, Phys. Status Solidi B, 2012, 249, 2057 CrossRef CAS
.
- J. M. Ribó, C. Blanco, J. Crusats, Z. El-Hachemi, D. Hochberg and A. Moyano, Chem. – Eur. J., 2014, 20, 17250 CrossRef PubMed
.
- S. Olsson, P. M. Björemark, T. Kokoli, J. Sundberg, A. Lennartson, C. J. McKenzie and M. Håkansson, Chem. – Eur. J., 2015, 21, 5211 CrossRef CAS PubMed
.
-
A. Guijarro and M. Yus, The Origin of Chirality in the Molecules of Life, Royal Society Chemistry, Cambridge, 2009 Search PubMed
.
- C. Viedma, Phys. Rev. Lett., 2005, 94, 065504 CrossRef PubMed
.
- V. A. Soloshonok, C. Roussel, O. Kitagawa and A. E. Sorochinsky, Chem. Soc. Rev., 2012, 41, 4180 RSC
.
- Y. Saito and H. Hyuga, Rev. Mod. Phys., 2013, 85, 603 CrossRef CAS
.
- K. Soai, S. Osanai, K. Kadowaki, S. Yonekubo, T. Shibata and I. Sato, J. Am. Chem. Soc., 1999, 121, 11235 CrossRef CAS
.
- T. Kawasaki, M. Sato, S. Ishiguro, T. Saito, Y. Morishita, I. Sato, H. Nishino, Y. Inoue and K. Soai, J. Am. Chem. Soc., 2005, 127, 3274 CrossRef CAS PubMed
.
- T. Kawasaki, Y. Matsumura, T. Tsutsumi, K. Suzuki, M. Ito and K. Soai, Science, 2009, 324, 492 CrossRef CAS PubMed
.
- K. Altwegg, H. Balsiger, A. Bar-Nun, J.-J. Berthelier, A. Bieler, P. Bochsler, C. Briois, U. Calmonte, M. R. Combi, H. Cottin, J. D. Keyser, F. Dhooghe, B. Fiethe, S. A. Fuselier, S. Gasc, T. I. Gombosi, K. C. Hansen, M. Haessig, A. Jäckel, E. Kopp, A. Korth, L. L. Roy, U. Mall, B. Marty, O. Mousis, T. Owen, H. Rème, M. Rubin, T. Sémon, C.-Y. Tzou, J. H. Waite and P. Wurz, Sci. Adv., 2016, 2, e160028 Search PubMed
.
- T. Kawasaki, M. Shimizu, D. Nishiyama, M. Ito, H. Ozawa and K. Soai, Chem. Commun., 2009, 4396 RSC
.
- S. L. Miller, Science, 1953, 117, 528 CrossRef CAS PubMed
.
- I. Weissbuch, L. Addadi, Z. Berkovitch-Yellin, E. Gati, M. Lahav and L. Leiserowitz, Nature, 1984, 310, 161 CrossRef CAS
.
- I. Weissbuch, L. Addadi, L. Leiserowitz and M. Lahav, J. Am. Chem. Soc., 1988, 110, 561 CrossRef CAS
.
- Y. Iitaka, Acta Crystallogr., 1961, 14, 1 CrossRef CAS
.
- K. Ishikawa, M. Tanaka, T. Suzuki, A. Sekine, T. Kawasaki, K. Soai, M. Shiro, M. Lahav and T. Asahi, Chem. Commun., 2012, 48, 6031 RSC
.
- A. V. Tarasevych, A. E. Sorochinsky, V. P. Kukhar, L. Toupet, J. Crassous and J.-C. Guillemin, CrystEngComm, 2015, 17, 1513 RSC
.
- K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767 CrossRef CAS
.
- T. Shibata, S. Yonekubo and K. Soai, Angew. Chem., Int. Ed., 1999, 38, 659 CrossRef CAS PubMed
.
- I. Sato, H. Urabe, S. Ishiguro, T. Shibata and K. Soai, Angew. Chem., Int. Ed., 2003, 42, 315 CrossRef CAS PubMed
.
- M. Avalos, R. Babiano, P. Cintas, J. L. Jimonez and J. C. Palacios, Chem. Commun., 2000, 887 RSC
.
- D. G. Blackmond, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5732 CrossRef CAS PubMed
.
- J. Podlech and T. Gehring, Angew. Chem., Int. Ed., 2005, 44, 5776 CrossRef CAS PubMed
.
- J. M. Brown, I. D. Gridnev and J. Klankermayer, Top. Curr. Chem., 2008, 284, 35 CrossRef CAS
.
- T. Gehring, M. Busch, M. Schlageter and D. Weingand, Chirality, 2010, 22, E173 CrossRef CAS PubMed
.
- B. Barabás, J. Tóth and G. Pályi, J. Math. Chem., 2010, 48, 457 CrossRef
.
- É. Dóka and G. Lente, J. Am. Chem. Soc., 2011, 133, 17878 CrossRef PubMed
.
-
J.-C. Micheau, C. Coudret and T. Buhse, Systems Chemistry in the Soai Reaction, in The Soai Reaction and Related Topic, ed. G. Pályi, C. Zucchi and L. Caglioti, Accad. Nazl. Sci. Lett. Arti, Editioni Artestampa, Modena, 2012, p. 169 Search PubMed
.
- A. J. Bissette and S. P. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800 CrossRef CAS PubMed
.
- O. Fülöp and B. Barabás, J. Math. Chem., 2016, 54, 10 CrossRef
.
- K. Soai and T. Kawasaki, Top. Curr. Chem., 2008, 284, 1 CrossRef CAS
.
- K. Soai, Proc. Jpn. Acad., Ser. B, 2019, 95, 89–110 CrossRef PubMed
.
- K. Soai, T. Kawasaki and A. Matsumoto, Acc. Chem. Res., 2014, 47, 3643 CrossRef CAS PubMed
.
-
K. Soai, A. Matsumoto and T. Kawasaki, Asymmetric Autocatalysis and the Origins of Homochirality of Organic Compounds. An Overview, in Advances in Asymmetric Autocatalysis and Related Topic, ed. G. Pályi, R. Kurdi and C. Zucchi, Elsevier Inc., Cambridge, 2017, ch. 1, p. 1 Search PubMed
.
- K. Soai, T. Kawasaki and A. Matsumoto, Tetrahedron, 2018, 74, 1973 CrossRef CAS
.
- D. G. Blackmond, C. R. McMillan, S. Ramdeehul, A. Schorm and J. M. Brown, J. Am. Chem. Soc., 2001, 123, 10103 CrossRef CAS PubMed
.
- I. D. Gridnev, J. M. Serafimov and J. M. Brown, Angew. Chem., Int. Ed., 2004, 43, 4884 CrossRef CAS PubMed
.
- L. Schiaffino and G. Ercolani, Angew. Chem., Int. Ed., 2008, 47, 6832 CrossRef CAS PubMed
.
- G. Ercolani and L. Schiaffino, J. Org. Chem., 2011, 76, 2619 CrossRef CAS PubMed
.
- M. Quaranta, T. Gehring, B. Odell, J. M. Brown and D. G. Blackmond, J. Am. Chem. Soc., 2010, 132, 15104 CrossRef CAS PubMed
.
- T. Gehring, M. Quaranta, B. Odell, D. G. Blackmond and J. M. Brown, Angew. Chem., Int. Ed., 2012, 51, 9539 CrossRef CAS PubMed
.
- I. Sato, D. Omiya, H. Igarashi, K. Kato, Y. Ogi, K. Tsukiyama and K. Soai, Tetrahedron: Asymmetry, 2003, 14, 975 CrossRef CAS
.
- A. Matsumoto, T. Abe, A. Hara, T. Tobita, T. Sasagawa, T. Kawasaki and K. Soai, Angew. Chem., Int. Ed., 2015, 54, 15218 CrossRef CAS PubMed
.
- T. Kawasaki, K. Jo, H. Igarashi, I. Sato, M. Nagano, H. Koshima and K. Soai, Angew. Chem., Int. Ed., 2005, 44, 2774 CrossRef CAS PubMed
.
- T. Kawasaki, K. Suzuki, K. Hatase, M. Otsuka, H. Koshima and K. Soai, Chem. Commun., 2006, 1869 RSC
.
- T. Kawasaki, H. Tanaka, T. Tsutsumi, T. Kasahara, I. Sato and K. Soai, J. Am. Chem. Soc., 2006, 128, 6032 CrossRef CAS PubMed
.
- T. Kawasaki, K. Suzuki, Y. Hakoda and K. Soai, Angew. Chem., Int. Ed., 2008, 47, 496 CrossRef CAS PubMed
.
- A. Matsumoto, Y. Kaimori, M. Uchida, H. Omori, T. Kawasaki and K. Soai, Angew. Chem., Int. Ed., 2017, 56, 545 CrossRef CAS PubMed
.
- T. Kawasaki, Y. Okano, E. Suzuki, S. Takano, S. Oji and K. Soai, Angew. Chem., Int. Ed., 2011, 50, 8131 CrossRef CAS PubMed
.
- A. Matsumoto, H. Ozaki, S. Harada, K. Tada, T. Ayugase, H. Ozawa, T. Kawasaki and K. Soai, Angew. Chem., Int. Ed., 2016, 55, 15246 CrossRef CAS PubMed
.
- D. J. Carter, B. Kahr and A. L. Rohl, Theor. Chem. Acc., 2012, 131, 1125 Search PubMed
.
- For the computational simulations using a chiral crystal of achiral N-(2-thienylcarbonyl)glycine, see: D. J. Carter, A. L. Rohl, A. Shtukenberg, S. D. Bian, C.-H. Hu, L. Baylon, B. Kahr, H. Mineki, K. Abe, T. Kawasaki and K. Soai, Cryst. Growth Des., 2012, 12, 2138 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedure for asymmetric autocatalysis; and analysis of the absolute handedness of γ-glycine. See DOI: 10.1039/c9ob00345b |
| ‡ Present address: Department of Chemistry, Biology and Environmental Science, Nara Women's University, Kita-Uoya Nishi-machi, Nara 630-8506, Japan. |
| This journal is © The Royal Society of Chemistry 2019 |