Gold catalysed transformation of 2-arylindoles to terphenyl amines via 3-dienyl indoles and Brønsted acid promoted formation of 2-carboxyindoles to 3-indenylindoles via 3-allenylindoles†
Abstract
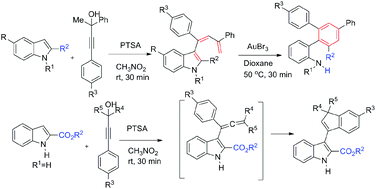
Gold-catalysed intramolecular reaction of unprotected/protected 3-dienyl indoles leads to the formation of functionalised terphenylamines with high efficiency; this reaction can also be accomplished in one pot by starting with 2-arylindole and propargylic alcohols. This reaction occurs via 3-dienyl indoles and proceeds likely by gold-catalysed cascade cyclisation, aromatisation and fragmentation of the indole heterocyclic moiety. We have also found that PTSA can work well giving comparable yields, but at least one mole equivalent of PTSA is required. On the other hand, 3-indenylindoles have been synthesised from indole-2-carboxylates (2-carboxyindoles) and propargylic alcohols through Brønsted acid mediation. Many of these indenylindoles exhibit isomerism (probably diastereomers) in the solution state (1H NMR). Two examples of indolo-indenyl acetates have been prepared by reacting the above terphenylanilines with PhI(OAc)2. Key products are characterised by X-ray crystallography.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...