On water: iodine-mediated direct construction of 1,3-benzothiazines from ortho-alkynylanilines by regioselective 6-exo-dig cyclization†
Abstract
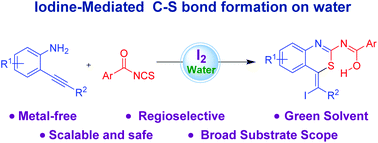
Herein, we report the 6-exo-dig ring closure of ortho-alkynylanilines with readily available aroyl isothiocyanate. An environmentally benign, metal- and base-free, iodine promoted cascade synthesis of highly functionalized (benzo[1,3]thiazin-2-yl)benzimidic acids has been accomplished via in situ generated ortho-alkynylthiourea. The established methodology employs the abundant chemical feedstock of ortho-alkynylanilines and aroyl isothiocyanates and could be applied in the late-stage synthesis of pharmaceutically active 1,3-benzothiazine containing molecules. Furthermore, the discovered protocol exclusively delivers bis (benzo[1,3]thiazin-2-yl)dibenzimidic acid products and preserves the iodo-olefin substitution pattern which can be exploited by further derivatization.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...