Open Access Article
Open Access ArticleAbsolute asymmetric Strecker synthesis in a mixed aqueous medium: reliable access to enantioenriched α-aminonitrile†
Shinobu
Miyagawa
a,
Shohei
Aiba
a,
Hajime
Kawamoto
a,
Yuji
Tokunaga
 a and
Tsuneomi
Kawasaki
a and
Tsuneomi
Kawasaki
 *b
*b
aDepartment of Materials Science and Engineering, University of Fukui, Bunkyo, Fukui, 910-8507, Japan
bDepartment of Applied Chemistry, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo, 162-8601, Japan. E-mail: tkawa@rs.tus.ac.jp
First published on 9th January 2019
Abstract
Without using chiral sources, the Strecker reaction of achiral hydrogen cyanide, p-tolualdehyde and benzhydrylamine gave enantioenriched L- or D-N-benzhydryl-α-(p-tolyl)glycine nitriles with up to >99% ee in a mixed solvent of water and methanol. Therefore, total spontaneous resolution of α-aminonitriles could occur through a prebiotic mechanism of α-amino acid synthesis. Moreover, it was demonstrated that the repetition of partial dissolution and crystallization of a suspended conglomerate of aminonitrile under solution-phase racemization could generate the enantiomeric imbalance to afford, in combination with the amplification of chirality, an enantioenriched product in every case. Among the 73 experiments that were carried out, D- and L-enriched isomers occurred 36 and 37 times, respectively. This stochastic behavior, under achiral or racemic starting conditions, meets the requirements of the spontaneous absolute asymmetric Strecker synthesis. The implications of the present results for the origin of chirality of α-amino acids are discussed.
Introduction
One of the major interests in science is the origin and amplification of biological homochirality before the emergence of life;1,2 that is, the occurrence of an overwhelming predominance of L-amino acids and D-sugars in nature compared with their mirror image molecules. Significant enantiomeric enrichment of biological organic compounds is a signature of life, and can therefore be considered as one of the keys to understanding the origin of life.3,4 A main focus of research addresses the question of how the enantiomeric imbalance of the initial chiral compound arose from an achiral or racemic starting environment in the prebiotic world.Several theories on the origins of chirality have been examined.5–16 Among them, spontaneous absolute asymmetric synthesis17 has been linked to the generation of enantiomerically enriched compounds. In the homogeneous reaction, asymmetric autocatalysis of 5-pyrimidyl alkanol (Soai reaction)18–20 gave the product in nearly enantiomerically pure form without the use of any chiral sources.21–23 In the heterogeneous reaction, chiral crystallization including total spontaneous resolution24 and stereospecific solid-state reaction have been considered as candidates.13,25–27
Asymmetric amplifications, which can enhance an initially induced low enantiomeric excess (ee) to extremely high ee, are essential for the formation of homochirality of organic compounds.28,29 Solid-state ee could be generated and amplified by Viedma ripening5,30 of a suspended solid under solution-phase racemization, which is a safe method to access enantioenriched chiral organic compounds.31–34 In the homogeneous reactions, positive nonlinear effects in asymmetric catalyses35 have been observed, including asymmetric autocatalysis.36,37 Thus, self-disproportionation of enantiomers is a fundamental phenomenon to improve the enantiopurity of chiral compounds.38
Our research focus includes Strecker-type synthesis39–42 because this has been considered a possible prebiotic mechanism for α-amino acid synthesis since the seminal report on electric discharge experiments conducted under plausible prebiotic conditions.3,43 The linkage between the origin of chirality and the formation of prebiotic amino acids may constitute a novel approach that can be used to explain the biological homochirality seen in L-amino acids. Based on this concept, we have reported the spontaneous formation and amplification of enantioenriched α-aminonitriles forming conglomerates in methanol, in which the enantiomerically enriched product could be obtained 43 times out of a total of 160 experiments.39 The solid-state ee of α-aminonitriles could be enhanced significantly39,40 by Viedma ripening5,30–33 and its modified method, i.e., temperature cycle.34
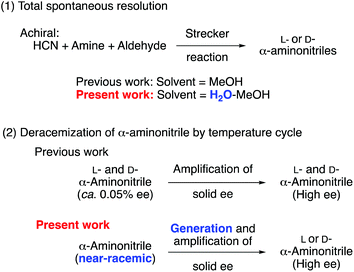
As shown in Fig. 1, the purpose of the present research was to examine (1) the absolute asymmetric Strecker synthesis44 (total spontaneous resolution) under aqueous conditions because water is a fundamental solvent in the synthesis of terrestrial prebiotic compounds and (2) deracemization of a near racemic conglomerate of α-aminonitrile45 by the temperature cycle to generate and amplify the solid-state chirality. These approaches would improve the frequency of the spontaneous generation of enantioenriched aminonitrile.
Here, we demonstrate that the Strecker reaction of three achiral reagents, hydrogen cyanide (HCN),4 amine and aldehyde, in a mixed aqueous medium, gives enantioenriched α-aminonitrile with high ee without using any chiral sources. In addition, a previously reported amplification cycle by temperature control40 could be used to induce the enantiomeric imbalances in the solid state to provide highly enantiomerically enriched aminonitriles, in which stochastic behavior was observed for the formation frequency of L- and D-enriched compounds.
Results and discussion
Generation of enantioenriched α-aminonitrile as a result of the Strecker reaction in a mixed aqueous medium
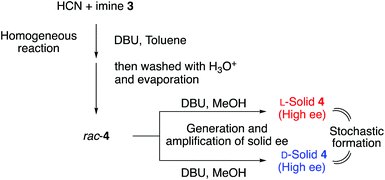
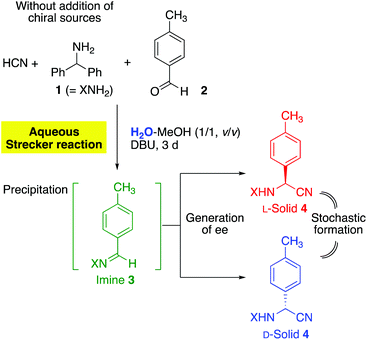
The Strecker reaction between HCN, benzhydrylamine (1), and p-tolualdehyde (2) was performed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and methanol in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (Fig. 2).46 The precipitation of imine 341 was observed from the homogeneous reaction mixture, and then the Strecker reaction proceeded gradually to afford α-aminonitrile 4 as a solid. Imine 3 disappeared from the solid phase over three days to give enantioenriched aminonitrile 4.
1 mixture of water and methanol in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (Fig. 2).46 The precipitation of imine 341 was observed from the homogeneous reaction mixture, and then the Strecker reaction proceeded gradually to afford α-aminonitrile 4 as a solid. Imine 3 disappeared from the solid phase over three days to give enantioenriched aminonitrile 4.
 | ||
| Fig. 2 Spontaneous formation of enantioenriched L- and D-α-aminonitrile 4 from the Strecker reaction of three achiral substrates in water–methanol. | ||
As shown in Table 1, L-product 4 was isolated as a solid in 65% yield with 99% ee (entry 1). In contrast, oppositely configured D-solid 4 was obtained in 55% yield with 53% ee (entry 2). It should be noted that aminonitrile 4 can be hydrolyzed under acidic conditions without a decrease in enantiopurity to give α-(p-tolyl)glycine with the absolute configuration corresponding to that of 4.39
| Entry | Solid α-aminonitrile 4 | |
|---|---|---|
| eeb/% (config.) | Yieldc/% | |
a The reaction was performed in a solution of 1 M DBU in water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The molar ratio used was HCN 1, v/v). The molar ratio used was HCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 1.5 2 = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (mmol).
b The ee value of the solid product, which was determined by high-performance liquid chromatography (HPLC) with a chiral stationary phase.
c The isolated yield of the crystalline product by filtration, which was calculated from the theoretical yield based on the molar amount of subjected 1 (the same molar amount as 2). The product in the filtrate was not included.
d Compound D-4 was isolated in 6% yield with 12% ee from the filtrate. 0.5 (mmol).
b The ee value of the solid product, which was determined by high-performance liquid chromatography (HPLC) with a chiral stationary phase.
c The isolated yield of the crystalline product by filtration, which was calculated from the theoretical yield based on the molar amount of subjected 1 (the same molar amount as 2). The product in the filtrate was not included.
d Compound D-4 was isolated in 6% yield with 12% ee from the filtrate.
|
||
| 1d | 99 (L) | 65 |
| 2 | 53 (D) | 55 |
| 3 | 1.0 (L) | 62 |
| 4 | 99 (L) | 57 |
| 5 | 99 (D) | 68 |
| 6 | 65 (L) | 60 |
| 7 | 84 (D) | 65 |
| 8 | 4.9 (D) | 42 |
| 9–23 | (Table S1†) | |
Among the 23 experiments (Table 1, entries 1–23) that were performed, crystallization of 4 occurred in every reaction and the distribution of the absolute handedness was approximately stochastic; i.e., D-4 occurred 11 times and the opposite L-isomer was isolated 12 times. Given that the reaction was initiated by mixing achiral reagents, the present observation constitutes one of the conditions necessary for spontaneous absolute asymmetric synthesis. Therefore, in combination with the hydrolysis, enantioenriched α-amino acids were obtained through a prebiotic mechanism.
Although the absolute ee values are widely distributed between 1.0 and 99% ee, a higher frequency of formation of 4 with high ee is observed compared with the previous method.39 The critical difference with respect to the present reactions is the precipitation of intermediate imine 3. It is considered that solid 3 suppresses the formation of 4 in the reaction equilibrium between 3 and 4, leading to the gradual formation of solid 4 based on the crystallinities of these compounds. After the formation of the primary nuclei of D- or L-aminonitrile 4, in principle with high ee, efficient dynamic resolution including amplification by the ripening may proceed to give enantioenriched 4.
The composition and ee of the suspended solid were assessed by analyzing the samples of the suspended solids (Table S2†). After 24 h, more than 95% of the solid was found to be imine 3. The molar ratio of 3/4 was 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 and the ee of 4 was 96% after two days, and finally the solid was composed almost entirely of 4 after three days. The final product 4 was isolated with 99% ee by filtration without a decrease in the initially observed 96% ee. Therefore, highly enantioselective reactive crystallization occurred in conjunction with the Strecker reaction.
62 and the ee of 4 was 96% after two days, and finally the solid was composed almost entirely of 4 after three days. The final product 4 was isolated with 99% ee by filtration without a decrease in the initially observed 96% ee. Therefore, highly enantioselective reactive crystallization occurred in conjunction with the Strecker reaction.
Frequent generation of enantioenriched p-tolylglycine nitrile 4 by repeated temperature cycles
The Strecker reaction was then conducted in methanol alone. Under these conditions, the three-component reaction proceeded and the spontaneous crystallization of 4 occurred within 1–12 h. Without DBU, the solubility of 4 decreased; thus, 4 precipitated out of every reaction mixture. Even if the initial solid-state ee was below the detectable level, highly efficient absolute asymmetric synthesis can be achieved if solid 4 can be enantioenriched by some method.As noted, we have reported a significant amplification of the solid-state ee by thermally controlled repetition of partial dissolution and crystallization.40 In our simulation, almost the same amount of L- and D-conglomerates 4 (racemate) was dissolved in the heating step; that is, the enantiomeric imbalance was concentrated in the residual solid 4. Subsequently, 4 was recrystallized at the gradual cooling step to keep the amplified (concentrated) ee of the residual 4, as rapid racemization occurred in the solution phase containing DBU. We postulate that this temperature cycle enhances the enantiomeric imbalance, even though the initial imbalance arises from a small fluctuation of the relative amount of enantiomorphs.
The suspension of near-racemic conglomerate 4 obtained from methanol was subjected to the cycles after the addition of DBU (Fig. 3A). As shown in Fig. 3B, generation and amplification of solid ee were monitored. In one reaction, an initial generation of L-enrichment (12% ee) was observed after three thermal cycles; the ee of L-4 was subsequently amplified to 26% ee and to 71% ee after the fourth and fifth cycles, respectively (Table 2, entry 1 and Fig. 3B). On the other hand, D-4 was obtained in 40% yield with 96% ee after five cycles through the enhancement of the initially observed 2.1% ee in the second cycle (entry 2 and Fig. 3B). Both L- and D-α-aminonitriles 4 were obtained with 10 and 3.1% ee values, respectively, after the amplification (entries 3 and 4). Thus, sufficient ee was generated during the cycles, which can be enhanced to high ee. The precipitation of imine 3 was not observed under these conditions.
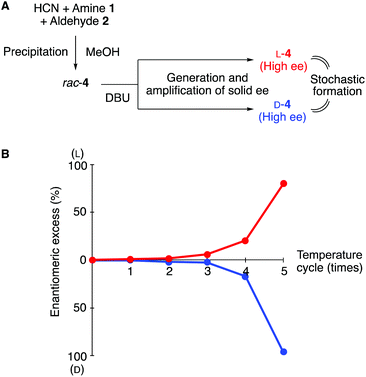 | ||
| Fig. 3 Spontaneous generation and amplification of ee in the Strecker reaction in methanol. (a) Schematic outline. (b) Generation and amplification of ee by the temperature cycles (see also Table S3†). | ||
| Entry | Solid α-aminonitrile 4 | Temperaturecycle/times | |
|---|---|---|---|
| eeb/% (config.) | Yieldc/% | ||
a The molar ratio used was HCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 1.65 2 = 1.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45 0.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45 (mmol).
b The ee was determined by using HPLC with a chiral stationary phase.
c The isolated yield of the crystalline product by filtration (see also Table 1 footnote c).
d Generation and amplification of ee were monitored as shown in Fig. 3B.
e After the precipitation of 4, the suspension was stirred for 13 h and concentrated in vacuo. The solid was then completely dissolved in CHCl3 and was separated in portions. After the removal of the solvent, a thermal cycle was applied to the suspension of the resulting solid 4 in 1 M DBU methanol solution, independently.
f After the precipitation of 4, the suspension was stirred for 15 h, then divided into portions, and then subjected to the procedure given in footnote e. The temperature cycle was then conducted independently. 0.45 (mmol).
b The ee was determined by using HPLC with a chiral stationary phase.
c The isolated yield of the crystalline product by filtration (see also Table 1 footnote c).
d Generation and amplification of ee were monitored as shown in Fig. 3B.
e After the precipitation of 4, the suspension was stirred for 13 h and concentrated in vacuo. The solid was then completely dissolved in CHCl3 and was separated in portions. After the removal of the solvent, a thermal cycle was applied to the suspension of the resulting solid 4 in 1 M DBU methanol solution, independently.
f After the precipitation of 4, the suspension was stirred for 15 h, then divided into portions, and then subjected to the procedure given in footnote e. The temperature cycle was then conducted independently.
|
|||
| 1d | 71 (L) | 38 | 5 |
| 2d | 96 (D) | 40 | 5 |
| 3 | 10 (L) | 46 | 4 |
| 4 | 3.1 (D) | 46 | 9 |
| 5e | 97 (L) | 69 | 11 |
| 6e | 93 (D) | 65 | 11 |
| 7f | 88 (L) | 65 | 8 |
| 8f | 91 (L) | 66 | 8 |
| 9f | 99 (L) | 68 | 12 |
| 10f | 99 (D) | 67 | 12 |
| 11–25 | (Table S4†) | ||
N-Benzhydryl-α-aminonitriles, formed from the corresponding aldehyde, have a racemization mechanism in only methanol47 that can proceed without bases such as DBU. Thus, another interesting issue is whether spontaneously precipitated solid 4 from methanol has an amplifiable solid-state ee. In our previous work, ca. 0.05% ee could be successfully amplified to >99.5% ee.40
Near-racemic solid 4, obtained from the same reaction, was separated into two portions and these were both subjected to the cycles independently (Table 2, entries 5 and 6). If there was amplifiable ee in the solid, the direction of the L/D-handedness should be the same after the amplification of each separated sample, we considered. As a result, L- and D-enantiomorphs 4 were obtained from independent cycles. Again, solid 4 was divided into four portions and subsequent amplification gave L-4 in three experimental runs (entries 7–9) and D-4 once (entry 10). Therefore, enough enantioenrichment, which can be amplified by the present method was not generated in the initial precipitation because of a much longer racemization half-life (t1/2) in the solution phase47 and rapid crystallization. It should be noted that nearly enantiomerically pure aminonitrile 4 can be synthesized by applying a sufficient number of temperature cycles, as shown in entries 9 and 10.
To check the distribution of the sense of asymmetry, the sequence of reactions was repeated (Table 2, entries 11–25). Among 25 experiments, D-4 occurred 12 times and L-4 formed 13 times, demonstrating that the absolute handedness seems to be stochastic. In addition, in the absence of chiral sources, an enantioenriched solid product was always obtained by this method. Therefore, repetition of temperature cycles can lead not only to asymmetric amplification but also to spontaneous generation of enantiomeric imbalances; in other words, the process can amplify a stochastic fluctuation of ee in the racemic conglomerate 4.
Finally, the generation of chirality was then examined starting from solid 4 prepared by the homogeneous Strecker reaction (see Scheme in Table 3). It may be conceivable that 4 formed from the homogeneous reaction without the addition of any chiral sources is more racemic than that formed through spontaneous crystallization. Additionally, aminonitrile 4 does not racemize in aprotic solvents such as toluene, ether and CHCl3. Thus, crystallization from these aprotic solvents may give more racemic conglomerates than those obtained through solution-phase racemization. Generation of solid-state chirality was examined starting from the assumed more racemic conglomerate 4 than that obtained from the heterogeneous Strecker reaction.
| Entry | Solid α-aminonitrile 4 | Temperaturecycle/times | |
|---|---|---|---|
| eea/% (config.) | Yieldb/% | ||
a The ee was determined by using HPLC with a chiral stationary phase.
b The isolated yield of the crystalline product by filtration.
c,d
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The Strecker reaction was performed in the same reaction vessel. After the removal of DBU, the solution was separated in portions and the solvent was removed. The suspension of resulting 4 was subjected to temperature cycles, independently. e The Strecker reaction was performed in the same reaction vessel. After the removal of DBU, the solution was separated in portions and the solvent was removed. The suspension of resulting 4 was subjected to temperature cycles, independently. e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Before temperature cycle, the homogeneous Strecker reaction was performed in mixed toluene/methanol solvent (1 Before temperature cycle, the homogeneous Strecker reaction was performed in mixed toluene/methanol solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) without DBU. 1, v/v) without DBU. |
|||
| 1 | 90 (D) | 49 | 5 |
| 2 | 79 (L) | 36 | 5 |
| 3c | 81 (D) | 55 | 11 |
| 4c | 90 (L) | 58 | 14 |
| 5d,e | 51 (D) | 52 | 9 |
| 6d,e | 75 (D) | 49 | 9 |
| 7d,e | 65 (L) | 49 | 14 |
| 8 | >99.5 (L) | 46 | 11 |
| 9–25 | (Table S5†) | ||
The Strecker reaction of imine 3 was performed in homogeneous toluene solution in the presence of a catalytic amount of DBU.48 After the completion of the reaction and removal of DBU, 4 was concentrated to form near racemic solid 4, which was suspended in 1 M DBU in methanol and subjected to the temperature cycles.
As shown in Table 3, D-4 was formed with 90% ee after five cycles (entry 1). In contrast, L-4 was isolated in 36% yield with 79% ee (entry 2). Therefore, starting from the racemic conglomerate of aminonitrile 4 obtained from the homogeneous Strecker reaction, the present method induced an ee and give enantioenriched L- or D-4. As shown in entries 3 and 4, when 4 obtained from the same reaction was divided into two portions, the composition of the resulting 4 became L- and D-4 with 81 and 90% ee, respectively. Again, when near rac-4 was divided into three portions (entries 5–7), D-4 occurred twice and L-4 once. It was possible to enhance the solid-state ee to near enantiopure by applying a sufficient number of cycles (entry 8).
To examine the distribution of the sense of chirality, additional experiments were conducted (Table 3, entries 9–25). Among 25 experiments, D-4 occurred 13 times and L-4 was obtained 12 times. Therefore, the observed stochastic outcomes of the molecular handedness suggest that these conditions enable absolute asymmetric synthesis.
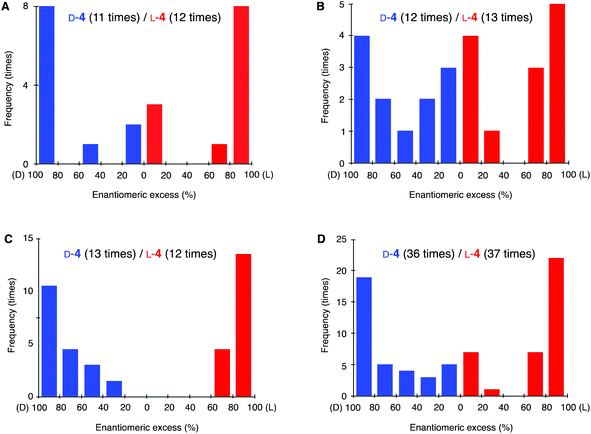
The distributions of absolute configuration and ee of 4 obtained from each method are summarized as histograms in Fig. 4. The absolute configurations seemed to be approximately stochastic under each of the three conditions; namely, the Strecker reaction in the mixed solvent of water and methanol (Fig. 4A), heterogeneous reaction (Fig. 4B), and homogeneous reaction (Fig. 4C). The overall experimental results are summarized in Fig. 4D. Among 73 experiments, enantioenriched product 4 could be obtained in every case; enantiomer D-4 occurred in 36 experiments and L-4 in 37 experiments. Thus, the sequence of the reaction seems to proceed without the intervention of external chiral effects. Absolute asymmetric synthesis was thereby realized by the simple control of the temperature of the reaction mixture. Although the distribution of ee values of solid aminonitriles 4 were varied between three methods (Fig. 3A–C), it should be noted that the value can be increased to >99.5% ee by applying further temperature cycles.
Conclusions
We have demonstrated that the absolute asymmetric Strecker amino acid synthesis can be conducted under aqueous conditions starting from achiral conditions to afford enantioenriched aminonitriles in the solid state. Even though the reactions started from near-racemic aminonitriles prepared from heterogeneous and homogeneous conditions, the present method induced an initial asymmetry that was enhanced significantly during subsequent amplification. By simple thermal control of the reaction suspension, highly enantioenriched chiral intermediates of α-amino acids with either L- or D-configurations could be generated in every case. The observed stochastic behaviour for the formation of L- or D-molecular handedness indicates that the process constitutes one of the conditions necessary for spontaneous absolute asymmetric synthesis. The present results increase the efficiency of the Strecker reactions as a prebiotic mechanism of amino acid synthesis to expand to the asymmetric synthesis of α-amino acids that is compatible with a prebiotic environment.Experimental
p-Tolualdehyde, benzhydrylamine, DBU, and methanol from commercial sources were used after distillation. Commercial distilled water was directly used for the reaction. Hydrogen cyanide (CAUTION!) was prepared from H2SO4 and NaCN in water and isolated by distillation (dried over CaCl2).Absolute asymmetric Strecker reaction (Table 1, entry 1)
To a solution of amine 1 (0.5 mmol, 87 μL), aldehyde 2 (0.5 mmol, 59 μL), and DBU (0.4 mL) in methanol (1 mL) and water (1 mL) was added HCN (1.5 mmol, 60 μL) at room temperature. After gentle stirring for 3 days, the resulting solid was collected by filtration and passed through silica gel using a mixture of ether/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) as an eluent to afford L-4 (0.33 mmol, 102 mg, 65% yield) with 99% ee. After dilution with ether, the filtrate was washed with 1 M aqueous HCl, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography using ether/hexane (1
3, v/v) as an eluent to afford L-4 (0.33 mmol, 102 mg, 65% yield) with 99% ee. After dilution with ether, the filtrate was washed with 1 M aqueous HCl, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography using ether/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) as an eluent to afford D-4 (9.5 mg, 0.03 mmol, 6% yield) with 12% ee.
3, v/v) as an eluent to afford D-4 (9.5 mg, 0.03 mmol, 6% yield) with 12% ee.
Strecker reaction in methanol followed by temperature cycles (Table 2, entry 1)
To a solution of amine 1 (0.45 mmol, 78 μL) and aldehyde 2 (0.45 mmol, 53 μL) in methanol (1 mL) was added HCN (1.65 mmol, 65 μL) at room temperature with stirring. After the precipitation of 4, DBU (0.2 mL) was added. The suspension was heated at 45–55 °C to dissolve ca. 90% of the solid, and then cooled slowly to room temperature over a period of ca. 1 h. Partial dissolution by heating and recrystallization by cooling (temperature cycle) were conducted five times. After the filtration of the mixture, the solid was washed with a small amount of methanol to afford L-4 (55 mg, 0.171 mmol, 38% yield) with 71% ee.Strecker reaction in homogeneous solution followed by temperature cycles (Table 3, entry 1)
To a solution of imine 3 (2.74 g, 9.6 mmol) and DBU (0.072 mL, 0.48 mmol) in toluene (30 mL) was added HCN (1.2 mL, 30.5 mmol) at room temperature. After stirring overnight, the reaction was quenched with 1 M aqueous HCl (15 mL) and the mixture was extracted three times with ether. The organic phase was purified by silica gel column chromatography using ether/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) as an eluent to afford 3 (9.4 mmol, 98% yield), which was dissolved in CHCl3 and a part of the solution was moved to a vial and concentrated in vacuo to afford solid 4 (173 mg, 0.55 mmol), which was suspended in methanol (1 mL). DBU (0.2 mL) and HCN (36 μL) were then added and temperature cycles were applied five times as described above. After the filtration of the reaction mixture, the resulting solid was washed with a small amount of methanol to afford D-4 (84.2 mg, 0.27 mmol, 49% yield) with 90% ee.
3, v/v) as an eluent to afford 3 (9.4 mmol, 98% yield), which was dissolved in CHCl3 and a part of the solution was moved to a vial and concentrated in vacuo to afford solid 4 (173 mg, 0.55 mmol), which was suspended in methanol (1 mL). DBU (0.2 mL) and HCN (36 μL) were then added and temperature cycles were applied five times as described above. After the filtration of the reaction mixture, the resulting solid was washed with a small amount of methanol to afford D-4 (84.2 mg, 0.27 mmol, 49% yield) with 90% ee.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the JSPS KAKENHI Grant Numbers JP16K05692, JP17J06244 and JP17H00312. T. K. thanks the Takeda Science Foundation, S. A. thanks the JSPS Research Fellowships for Young Scientists.Notes and references
- J. F. Bada, Nature, 1995, 374, 594 CrossRef CAS.
- M. Bolli, R. Micura and A. Eschenmoser, Chem. Biol., 1997, 4, 309–320 CrossRef CAS PubMed.
- S. L. Miller, Science, 1953, 117, 528–529 CrossRef CAS PubMed.
- J. D. Sutherland, Angew. Chem., Int. Ed., 2016, 55, 104–121 CrossRef CAS.
- C. Viedma, Phys. Rev. Lett., 2005, 94, 065504 CrossRef PubMed.
- Y. Inoue, Chem. Rev., 1992, 92, 741–770 CrossRef CAS.
- S. Pizzarello and A. L. Weber, Science, 2004, 303, 1151 CrossRef CAS.
- M. Klussmann, H. Iwamura, S. P. Mathew, D. H. Wells Jr., U. Pandya, A. Armstrong and D. G. Blackmond, Nature, 2006, 441, 621–623 CrossRef CAS PubMed.
- Y. Hayashi, M. Matsuzawa, J. Yamaguchi, S. Yonehara, Y. Matsumoto, M. Shoji, D. Hashizume and H. Koshino, Angew. Chem., Int. Ed., 2006, 45, 4593–4597 CrossRef CAS PubMed.
- R. Breslow and M. S. Levine, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12979 CrossRef CAS PubMed.
- U. J. Meierhenrich, L. Nahon, C. Alcaraz, J. H. Bredehöft, S. V. Hoffmann, B. Barbier and A. Brack, Angew. Chem., Int. Ed., 2005, 44, 5630–5634 CrossRef CAS PubMed.
- J. M. Ribo, J. Crusats, F. Sagues, J. Claret and R. Rubires, Science, 2001, 292, 2063–2066 CrossRef CAS.
- I. Weissbuch and M. Lahav, Chem. Rev., 2011, 111, 3236–3267 CrossRef CAS.
- D. K. Kondepudi, R. J. Kaufman and N. Singh, Science, 1990, 250, 975–976 CrossRef CAS PubMed.
- K. Soai, S. Osanai, K. Kadowaki, S. Yonekubo, T. Shibata and I. Sato, J. Am. Chem. Soc., 1999, 121, 11235–11236 CrossRef CAS.
- T. Kawasaki, M. Sato, S. Ishiguro, T. Saito, Y. Morishita, I. Sato, H. Nishino, Y. Inoue and K. Soai, J. Am. Chem. Soc., 2005, 127, 3274–3275 CrossRef CAS PubMed.
- K. Mislow, Collect. Czech. Chem. Commun., 2003, 68, 849–864 CrossRef CAS.
- K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767–768 CrossRef CAS.
- K. Soai, T. Kawasaki and A. Matsumoto, Acc. Chem. Res., 2014, 47, 3643–3654 CrossRef CAS PubMed.
- K. Soai, T. Kawasaki and A. Matsumoto, Tetrahedron, 2018, 74, 1973–1990 CrossRef CAS.
- K. Soai, I. Sato, T. Shibata, S. Komiya, M. Hayashi, Y. Matsueda, H. Imamura, T. Hayase, H. Morioka, H. Tabira, J. Yamamoto and Y. Kowata, Tetrahedron: Asymmetry, 2003, 14, 185–188 CrossRef CAS.
- T. Kawasaki, K. Suzuki, M. Shimizu, K. Ishikawa and K. Soai, Chirality, 2006, 18, 479–482 CrossRef CAS PubMed.
- K. Suzuki, K. Hatase, D. Nishiyama, T. Kawasaki and K. Soai, J. Syst. Chem., 2010, 1, 5 CrossRef CAS.
- E. Havinga, Biochim. Biophys. Acta, 1954, 13, 171–174 CrossRef CAS.
- (a) F. Yagishita, H. Ishikawa, T. Onuki, S. Hachiya, T. Mino and M. Sakamoto, Angew. Chem., Int. Ed., 2012, 51, 13023–13025 CrossRef CAS PubMed; (b) N. Uemura, S. Toyoda, H. Ishikawa, Y. Yoshida, T. Mino, Y. Kasashima and M. Sakamoto, J. Org. Chem., 2018, 83, 9300–9304 CrossRef CAS PubMed.
- P. M. Björemark, S. Olsson, T. Kokoli and M. Håkansson, Chem. – Eur. J., 2015, 21, 8750–8753 CrossRef PubMed.
- T. Matsuura and H. Koshima, J. Photochem. Photobiol., C, 2005, 6, 7–24 CrossRef CAS.
- M. M. Green, J.-W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. B. Selinger and J. V. Selinger, Angew. Chem., Int. Ed., 1999, 38, 3138–3154 CrossRef.
- T. Kawasaki, Y. Matsumura, T. Tsutsumi, K. Suzuki, M. Ito and K. Soai, Science, 2009, 324, 492–495 CrossRef CAS PubMed.
- Z. El-Hachemi, J. Crusats, J. M. Ribó, J. M. McBride and S. V. Verdaguer, Angew. Chem., Int. Ed., 2011, 50, 2359–2363 CrossRef CAS PubMed.
- (a) W. L. Noorduin, T. Izumi, A. Millemaggi, M. Leeman, H. Meekes, W. J. P. Van Enckevort, R. M. Kellogg, B. Kaptein, E. Vlieg and D. G. Blackmond, J. Am. Chem. Soc., 2008, 130, 1158–1159 CrossRef CAS PubMed; (b) C. Viedma, J. E. Ortiz, T. de Torres, T. Izumi and D. G. Blackmond, J. Am. Chem. Soc., 2008, 130, 15274–15275 CrossRef CAS PubMed.
- W. L. Noorduin, A. A. C. Bode, M. van der Meijden, H. Meekes, A. F. van Etteger, W. J. P. van Enckevort, P. C. M. Christianen, B. Kaptein, R. M. Kellogg, T. Rasing and E. Vlieg, Nat. Chem., 2009, 1, 729–732 CrossRef CAS PubMed.
- R. R. E. Steendam, J. M. M. Verkade, T. J. B. van Benthem, H. Meekes, W. J. P. van Enckevort, J. Raap, F. P. J. T. Rutjes and E. Vlieg, Nat. Commun., 2014, 5, 5543 CrossRef CAS PubMed.
- K. Suwannasang, A. E. Flood, C. Rougeot and G. Coquerel, Cryst. Growth Des., 2013, 13, 3498–3504 CrossRef CAS.
- T. Satyanarayana, S. Abraham and H. B. Kagan, Angew. Chem., Int. Ed., 2009, 48, 456–494 CrossRef CAS.
- I. Sato, H. Urabe, S. Ishiguro, T. Shibata and K. Soai, Angew. Chem., Int. Ed., 2003, 42, 315–317 CrossRef CAS PubMed.
- G. Storch and O. Trapp, Nat. Chem., 2017, 9, 179–187 CrossRef CAS PubMed.
- J. Han, O. Kitagawa, A. Wzorek, K. D. Klika and V. A. Soloshonok, Chem. Sci., 2018, 9, 1718–1739 RSC.
- T. Kawasaki, N. Takamatsu, S. Aiba and Y. Tokunaga, Chem. Commun., 2015, 51, 14377–14380 RSC.
- S. Aiba, N. Takamatsu, T. Sasai, Y. Tokunaga and T. Kawasaki, Chem. Commun., 2016, 52, 10834–10837 RSC.
- S. Miyagawa, K. Yoshimura, Y. Yamazaki, N. Takamatsu, T. Kuraishi, S. Aiba, Y. Tokunaga and T. Kawasaki, Angew. Chem., Int. Ed., 2017, 56, 1055–1058 CrossRef CAS PubMed.
- N. Takamatsu, S. Aiba, T. Yamada, Y. Tokunaga and T. Kawasaki, Chem. – Eur. J., 2018, 24, 1304–1310 CrossRef CAS PubMed.
- E. T. Parker, M. Zhou, A. S. Burton, D. P. Glavin, J. P. Dworkin, R. Krishnamurthy, F. M. Fernández and J. L. Bada, Angew. Chem., Int. Ed., 2014, 53, 8132–8136 CrossRef CAS PubMed.
- For the aqueous catalytic asymmetric Strecker reaction, see: S. J. Zuend, M. P. Coughlin, M. P. Lalonde and E. N. Jacobsen, Nature, 2009, 461, 968–970 CrossRef CAS PubMed.
- For a recent example of deracemization of the conglomerate of aminonitriles, see: I. Baglai, M. Leeman, K. Wurst, B. Kaptein, R. M. Kellogg and W. L. Noorduin, Chem. Commun., 2018, 54, 10832–10834 RSC.
- Substrates 1 and 2 could not dissolve completely in the mixed solvent with a higher content ratio of water than 50%.
- The racemization half-life (t1/2) of 4 was measured to be ca. 9 h in methanol (0.6 mM) and 0.5 min in 1 M DBU in methanol (12.5 mM). For t1/2 of a related compound, see: V. Banphavichit, W. Mansawat, W. Bhanthumnavin and T. Vilaivan, Tetrahedron, 2009, 65, 5849–5854 CrossRef CAS.
- Without DBU, the Strecker reaction between 3 and HCN in toluene took over days and the reaction stopped without completion.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental results. See DOI: 10.1039/c8ob03092h |
| This journal is © The Royal Society of Chemistry 2019 |