Understanding the effects of ionic liquids on a unimolecular substitution process: correlating solvent parameters with reaction outcome†
Abstract
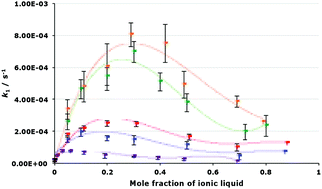
A unimolecular substitution process was studied in five different ionic liquids, with systematic variation of either the cation or anion, in order to determine the factors leading to the increase in the rate constant for the process relative to acetonitrile. It was found that both components of the ionic liquid, and the proportion of the salt in the reaction mixture, affect the rate constant. Activation parameters determined for the process suggest that there is a balance between interactions of the components of the ionic liquid with both starting material and transition state. A correlation was found between the rate constant and a combination of Kamlet–Taft solvent parameters; with the polarisability of the solvent being the most significant factor. As this reaction proceeds through both unimolecular and bimolecular pathways, competition experiments determined that the unimolecular pathway for the reaction can be favoured using small amounts of ionic liquid in the reaction mixture, demonstrating the potential to control reaction mechanisms using ionic liquids.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...