Metal-free photocatalytic thiol–ene/thiol–yne reactions†
Abstract
The organic photocatalyst (9-mesityl-10-methylacridinum tetrafluoroborate) in the presence of visible light is used to initiate thiol–ene and thiol–yne reactions. Thiyl radicals are generated upon quenching the photoexcited catalyst with a range of thiols. The highlighted mild nature of the reaction conditions allows a broad substrate scope of the reactants. Relying on this efficient metal-free condition, both thiol–ene and thiol–yne reactions between carbohydrates and peptides could be realized in excellent yields.
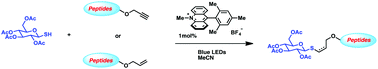
- This article is part of the themed collections: Synthetic methodology in OBC and New Talent


 Please wait while we load your content...
Please wait while we load your content...