Synthesis of (Z)-β-halo α,β-unsaturated carbonyl systems via the combination of halotrimethylsilane and tetrafluoroboric acid†
Abstract
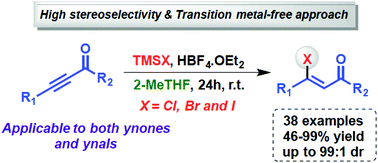
A convenient and broadly applicable method for the hydrohalogenation of ynones is described, by the combination of halotrimethylsilanes and tetrafluoroboric acid. Practically, one equivalent of HX (Brønsted acid) and BF3 (Lewis acid) is smoothly generated, which activates the carbonyl compounds. Through this protocol, 42 examples of (Z)-β-halovinyl carbonyl compounds (Cl, Br and I) were obtained, in good yields and high stereoselectivity having 2-MeTHF as a solvent.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...