Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Peptide-directed encapsulation of inorganic nanoparticles into protein containers
Matthias
Künzle
,
Johanna
Mangler
,
Marcel
Lach
and
Tobias
Beck
*
RWTH Aachen University, Institute of Inorganic Chemistry, JARA-SOFT (Researching Soft Matter), and I3TM, 52074 Aachen, Germany. E-mail: tobias.beck@ac.rwth-aachen.de
First published on 19th March 2019
Abstract
Correction for ‘Peptide-directed encapsulation of inorganic nanoparticles into protein containers’ by Tobias Beck et al., Nanoscale, 2018, 10, 22917–22926.
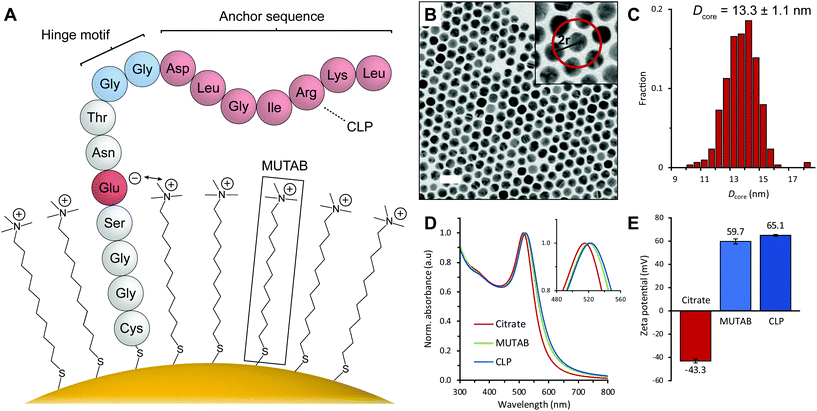
The authors have noticed that the peptide sequence in Fig. 2A in the originally published article was incorrect. The second aspartic acid in the anchor sequence should have instead been a glycine. A corrected version of Fig. 2 is provided below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2019 |