In situ encapsulation of Co-based nanoparticles into nitrogen-doped carbon nanotubes-modified reduced graphene oxide as an air cathode for high-performance Zn–air batteries†
Haocheng
Qi‡
a,
Yingying
Feng‡
a,
Zhenzhen
Chi‡
a,
Yuanyuan
Cui
b,
Minghui
Wang
a,
Jie
Liu
 ac,
Ziyang
Guo
ac,
Ziyang
Guo
 *a,
Lei
Wang
*a,
Lei
Wang
 *a and
Shouhua
Feng
a
*a and
Shouhua
Feng
a
aState Key Laboratory of Eco-chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China. E-mail: zyguo@qust.edu.cn; inorchemwl@126.com; Fax: +86-21-51630318; Tel: +86-21-51630318
bShimadzu China Co. LTD, Shanghai 200233, China
cGuangxi Key Laboratory for Electrochemical Energy Materials, Guangxi University, P. R. China
First published on 23rd October 2019
Abstract
Exploring highly efficient catalysts for the oxygen reduction/evolution reaction (ORR/OER) is very important in rechargeable Zn–air batteries. N-doped carbon coupled with transition metal-based species are among the most promising cathode catalysts for Zn–air batteries. However, the aggregation of metal-based sites during the synthetic/cycling process is a serious drawback of these catalysts. Herein, in situ encapsulation of ultra-small Co/Co4N nanoparticles into N-doping carbon nanotubes (N-CNTs) anchored on reduced GO (Co/Co4N@N-CNTs/rGO) has been achieved through pyrolyzing a core–shell-structured ZIF-8@ZIF-67-modified GO (ZIF-8@ZIF-67/GO) precursor; the nanoparticles have been further applied as a bifunctional catalyst in Zn–air batteries. Benefitting from its uniform dispersion of Co-based particles, close contact of Co/Co4N species and N-CNTs, and high N content, Co/Co4N@N-CNTs/rGO shows outstanding catalytic activity/stability towards ORR and OER. Moreover, Zn volatilization and rGO introduction in Co/Co4N@N-CNTs/rGO can effectively promote the reactions of Zn–air cells. Hence, the Co/Co4N@N-CNTs/rGO-based conventional Zn–air battery exhibits a fantastic specific capacity of 783 mA h gZn−1, a continuous discharge platform over 6 days, a high-power density of ∼200 mW cm−2 and an ultra-long cycling life of 440 h with a small overpotential of ∼0.8 V. Moreover, a flexible Co/Co4N@N-CNTs/rGO-based Zn–air cell was also designed and revealed outstanding mechanical flexibility and good electrochemical performance, which suggests its potential application prospects in wearable electronic devices.
Introduction
With the rapid consumption of fossil fuels and the obviously increased demand for clean energy, developing effective, low-cost, clean, safe and sustainable energy conversion/storage devices is extremely critical in modern society.1–8 Rechargeable lithium-ion batteries are considered to be effective energy conversion systems; they have been used in portable electronics and electric transports.9 However, the further development of lithium-ion batteries is hindered by the limited supply of metallic lithium and their unsatisfactory energy density.10 Rechargeable metal–air batteries have attracted intensive interest due to their higher energy density compared with lithium-ion batteries.11–13 Among these, Zn–air batteries have many benefits (e.g. low cost, safety, super-high theoretical energy density (1086 W h kg−1) and environmental benignity); thus, they are among the most promising energy storage systems.14 It is well known that the two most important electrochemical processes occurring at the air cathodes in Zn–air batteries are the oxygen reduction reaction (ORR) during the discharge process and the oxygen evolution reaction (OER) during the charge process.15,16 However, the kinetic rate of the OER/ORR process is very slow in Zn–air batteries, which results in high overpotential, inferior rechargeability, and low energy efficiency.17,18 Recent reports have demonstrated that precious metal-based materials (e.g. Pt, Ru, Ir, RuO2, and IrO2) can effectively accelerate the ORR and OER due to their superior catalytic activity.19–24 However, the high cost, poor stability and scarcity of noble metal-based catalysts seriously impact their practical application in Zn–air batteries.25 Therefore, developing excellent bifunctional catalysts for Zn–air batteries with low cost and good durability is highly urgent.Nitrogen (N)-doping carbon materials, such as N-doped mesoporous carbon, N-doped graphene and N-doped nanoporous carbon fiber/CNTs, have been widely studied as alternative candidates to noble metal-based catalysts because these N-doped carbon catalysts not only exhibit superior catalytic activity towards ORR, comparable to that of commercial Pt/C, but also have much higher ORR stability than precious metal-based materials.26–30 However, the OER activities of these N-doped carbon catalysts are usually unsatisfactory. On the other hand, transition metal-containing materials, especially Co-based oxides, sulfides, nitrides, carbides, hydroxides, etc., have been demonstrated to exhibit superb OER performance which is even better than those of commercial IrO2 and RuO2-based materials.31–38 Therefore, many recent studies have focused on developing transition metal (TM)-based nanoparticles integrated with N-doped carbon matrices (NC) as bifunctional catalysts for Zn–air batteries.39–41 One of the simplest and most efficient methods for preparing TM-NC catalysts among the above reports is the carbonization of N-containing metal–organic frameworks (MOFs, especially zeolitic-imidazole frameworks (ZIFs)) because the metal ions and N-containing organic linkers in these ZIFs can be directly transformed into TM-based active sites with NCs during the pyrolysis process.42,43 Moreover, the TM-NCs derived from ZIFs can maintain many merits of their corresponding precursors, such as plentiful pores and firm frameworks; thus, they have been widely investigated as effective bifunctional catalysts in Zn–air batteries.44–46 However, there are still many drawbacks of these ZIF-derived materials that negatively affect their OER/ORR activity and stability, such as aggregation of the metal-based nodes, low graphitization extent, limited surface area, and poor carbon/active metal contact. Hence, developing novel ZIF-derived nanocomposites which can overcome the above disadvantages should be an important issue for the development of Zn–air batteries.
Herein, we firstly modified graphene oxide (GO) with ZIF-67 coated on ZIF-8 (ZIF-8@ZIF-67) and further used the resulting ZIF-8@ZIF-67-modified GO (ZIF-8@ZIF-67/GO) as a precursor to synthesize in situ encapsulations of ultra-small Co/Co4N nanoparticles into N-doped carbon nanotubes (N-CNTs)-modified reduced GO (Co/Co4N@N-CNTs/rGO). The as-prepared Co/Co4N@N-CNTs/rGO catalyst was further used as an air cathode for Zn–air cells. The typical core–shell structure of Co/Co4N@N-CNTs can effectively suppress the aggregation of Co/Co4N particles and improve the contact between the N-CNTs and Co-based species in Co/Co4N@N-CNTs/rGO. In addition, the evaporation of Zn after pyrolysis and the existence of the rGO matrix can obviously enhance the specific surface area of Co/Co4N@N-CNTs/rGO to ensure sufficient active space and superior ion/electron transfer for ORR/OER. Moreover, the high N content and the ultra-small Co/Co4N nanoparticles in Co/Co4N@N-CNTs/rGO can offer excellent catalytic activity towards the ORR and OER. Hence, the Co/Co4N@N-CNTs/rGO-based conventional Zn–air batteries show low voltage polarization, ultra-long cycling life and superior power density. More interestingly, flexible Zn–air batteries using Co/Co4N@N-CNTs/rGO cathodes have also been constructed; they show high mechanical strength and good electrochemical performance under a series of bending conditions.
Experimental section
Material synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, the as-prepared mixture was further carbonized at 900 °C for 2 h under flowing Ar gas. After cooling, in situ encapsulation of the ultra-small Co/Co4N nanoparticles into N-doped carbon nanotubes (N-CNTs)-modified reduced GO (denoted as Co/Co4N@N-CNTs/rGO) was achieved successfully. For comparison, the precursors ZIF-67, ZIF-8, ZIF-67-modified GO (ZIF-67/GO) and ZIF-8-modified GO (ZIF-8/GO) and their derivative encapsulations of Co/Co4N nanoparticles in N-doped carbon-modified reduced GO (denoted as Co/Co4N@NC/rGO) and N-doped carbon-modified reduced GO (denoted as NC/rGO) were also synthesized; the corresponding processes are given in the ESI.†
1. Then, the as-prepared mixture was further carbonized at 900 °C for 2 h under flowing Ar gas. After cooling, in situ encapsulation of the ultra-small Co/Co4N nanoparticles into N-doped carbon nanotubes (N-CNTs)-modified reduced GO (denoted as Co/Co4N@N-CNTs/rGO) was achieved successfully. For comparison, the precursors ZIF-67, ZIF-8, ZIF-67-modified GO (ZIF-67/GO) and ZIF-8-modified GO (ZIF-8/GO) and their derivative encapsulations of Co/Co4N nanoparticles in N-doped carbon-modified reduced GO (denoted as Co/Co4N@NC/rGO) and N-doped carbon-modified reduced GO (denoted as NC/rGO) were also synthesized; the corresponding processes are given in the ESI.†
Electrochemical measurements
Results and discussion
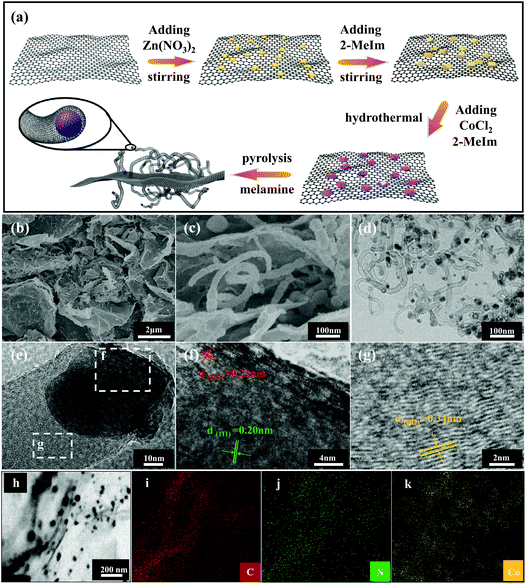
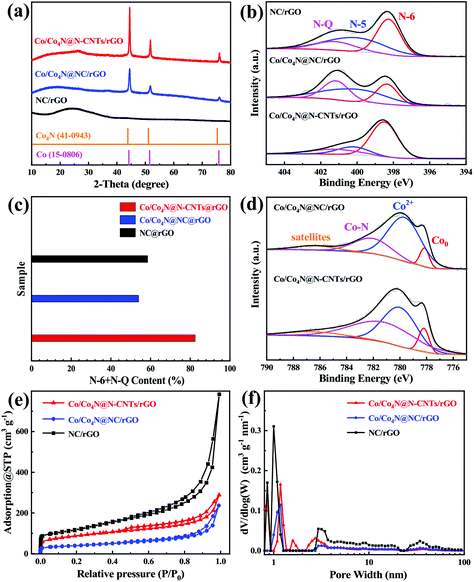
The synthesis process of in situ encapsulation of the ultra-small Co/Co4N nanoparticles into N-doped carbon nanotubes (N-CNTs)-modified reduced GO (Co/Co4N@N-CNTs/rGO) is illustrated in Fig. 1a. As shown in Fig. 1a, ZIF-8 nanocubes were synthesized through the seed-mediated growth reaction between Zn salts and 2-methylimidazole (2-MeIm) and were further uniformly in situ grown on both sides of the GO layer due to the electrostatic interactions between the electropositive Zn2+ and electronegative oxygen-based functional groups. Subsequently, the above resulting ZIF-8-coated GO (ZIF-8/GO) composite was evenly dispersed into a methanol solution by ultrasonic treatment. After adding CoCl2 and 2-MeIm, ZIF-67 was epitaxially grown on the surface of ZIF-8 nanocubes through a solvothermal reaction due to the coordination reaction between Co2+ and the surface MeIm of the ZIF-8 nanocubes. After that, the core-cell ZIF-8@ZIF-67 crystals-modified GO layer (ZIF-8@ZIF-67/GO) was successfully obtained. Interestingly, it was found that ZIF-8@ZIF-67/GO powder is black, which is different from ZIF-8 (white), ZIF-67 (purple), ZIF-8/GO (earthy yellow) and ZIF-67/GO (modena) (Fig. S1†). Then, the obtained ZIF-8@ZIF-67/GO was mixed with melamine and further pyrolyzed at 900 °C under Ar gas. During the pyrolyzation process, Co2+ and the GO layer in ZIF-8@ZIF-67/GO were reduced to metallic Co and rGO sheets, respectively, while the N-containing organic ligands and melamine in the precursor also changed into porous carbon nitride and N-doped carbon nanotubes (N-CNTs). Moreover, Zn2+ derived from ZIF-8 was firstly reduced to metallic Zn and then easily volatilized at 900 °C to produce abundant porous structures. Finally, ZIF-8@ZIF-67/GO was transformed into ultra-small Co/Co4N nanoparticles encapsulated into N-CNTs-modified rGO (Co/Co4N@N-CNTs/rGO). To clarify the morphology and structure characteristics of Co/Co4N@N-CNTs/rGO and the precursors, field emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM) measurements were conducted. GO shows a typical thin layer structure (Fig. S2†). It can be found from Fig. S3† that both sides of GO are tightly embedded with ZIF-8 nanocubes (ZIF-8/GO) with an average size of 150 nm, suggesting that the interaction of GO and ZIF-8 is very strong in ZIF-8/GO. In addition, Fig. S4† presents that the ZIF (i.e. ZIF-8@ZIF-67) particles anchored on the surface of the GO layer in ZIF-8@ZIF-67/GO exhibit a polyhedral morphology, which is obviously different from that of ZIF-8 in ZIF-8/GO (Fig. S3†). Moreover, the surface content of Zn element in ZIF-8@ZIF-67/GO is much lower than that in ZIF-8/GO (Fig. S3 and 4†). The above results further indicate that ZIF-67 is coated on the surface of ZIF-8 nanocubes in ZIF-8@ZIF-67/GO. After the carbonization process, the as-prepared Co/Co4N@N-CNTs/rGO shows a typical film structure with a rough surface (Fig. 1b). The enlarged SEM image of Co/Co4N@N-CNTs/rGO presents that numerous CNTs are evenly distributed on the surface of the rGO layers (Fig. 1c). In addition, the low-resolution TEM image shown in Fig. 1d indicates that there are many CNTs in Co/Co4N@N-CNTs/rGO and that some of them have wall thicknesses of 3 nm (Fig. S5†). In the enlarged TEM image of Co/Co4N@N-CNTs/rGO (Fig. 1e), it can be found that the Co-based nanoparticles are in situ encapsulated into the top of the CNTs, which can effectively inhibit the aggregation or exfoliation of Co-based nanoparticles during long-term cycling. Fig. 1f shows that there are two different visible lattice spacings in the Co-based nanoparticles: 0.23 and 0.20 nm, which may correspond to the (111) layer of Co4N and the (222) plane of metal Co, respectively. It can also be observed from the high resolution TEM image in Fig. 1g that the outside shell exhibits an interplanar distance of 0.34 nm, assigned to the (002) layer of graphite carbon. To further clarify the element composition/distribution of Co/Co4N@N-CNTs/rGO, energy-dispersive X-ray (EDX) mapping technology was used. The results shown in Fig. 1h–k indicate that C, N, and Co elements are evenly distributed in the skeleton of Co/Co4N@N-CNTs/rGO. Moreover, the morphologies of ZIF-67-coated GO (ZIF-67/GO) and the derivatives ZIF-8/GO and ZIF-67/GO were studied (Fig. S6–8†).The crystalline structures and phase evolutions of the as-prepared ZIF-8/GO, ZIF-67/GO, ZIF-8@ZIF-67/GO and their derivatives were analyzed by powder X-ray diffraction (XRD) characterization. It can be seen from Fig. S9† that the precursors ZIF-8/GO, ZIF-67/GO and ZIF-8@ZIF-67/GO have similar diffraction peaks, assigned to the typical ZIF-8 phase. Fig. 2a gives the XRD patterns of Co/Co4N@N-CNTs/rGO, Co/Co4N@NC/rGO, and NC/rGO. As shown in Fig. 2a, there are only two typical broad peaks around 26.5° and 42.8°, assigned to the (002) and (100) facets of graphitized carbon, in the XRD pattern of NC/rGO; this is due to the fact that the Zn phase in ZIF-8@GO is completely evaporated during the high-temperature pyrolysis process. However, both Co/Co4N@N-CNTs/rGO and Co/Co4N@NC/rGO show obvious peaks located at 44° and 52°, which can be attributed to Co (JCPDS 15-0806) and Co4N species (JCPDS 41-0943).47,48 X-ray photoelectron spectroscopy (XPS) technology was also used to detect the surface elemental states and chemical compositions of Co/Co4N@N-CNTs/rGO, Co/Co4N@NC/rGO and NC/rGO. Fig. 2b shows the high-resolution N 1s XPS spectra of the three annealed derivatives, which were deconvoluted into three peaks: pyridinic N (N-6, ∼398.4 eV), pyrrolic N (N-5, ∼400.2 eV) and graphitic N (N-Q, ∼401.2 eV). It has been demonstrated that N-6 and N-Q species are beneficial to ORR activity.49–51Fig. 2c shows that the surface content of the (N-6 + N-Q) phases in Co/Co4N@N-CNTs/rGO is 82.45%, which is obviously higher than those in Co/Co4N@NC/rGO (53.94%) and NC/rGO (58.5%). This phenomenon demonstrates that Co/Co4N@N-CNTs/rGO should show the best ORR performance among three derivatives. Moreover, there are four fixed peaks at around 778.2, 779.9, 782.1 and 786.4 eV in the high-resolution Co 2p2/3 XPS spectra of Co/Co4N@N-CNTs/rGO and Co/Co4N@NC/rGO (Fig. 2d), which indicate metallic Co, Co–O, Co–N and satellite phases, respectively. Moreover, Co–N species have been demonstrated to enhance OER activity.25,44 It can be further observed from Fig. S10† that the Co–N contents in Co/Co4N@N-CNTs/rGO and Co/Co4N@NC/rGO are 45.4% and 25.8%, which suggests superior catalytic activity of Co/Co4N@N-CNTs/rGO towards OER. The porous structures and pore-size distributions of Co/Co4N@N-CNTs/rGO, Co/Co4N@NC/rGO, and NC/rGO were also investigated by N2 adsorption/desorption isotherms. It can be found from Fig. 2e that all these derivatives exhibit distinct uptakes at low relative pressure (P/P0 < 0.05) and typical hysteresis loops in the high relative pressure range from 0.8 to 1.0, respectively, which indicates the co-existence of mesopores and micropores in these samples. Fig. 2f further indicates that there are two kinds of pores in the three ZIF-derived materials: micropores (∼1 nm) and mesopores (∼3 nm) (Fig. 2f).
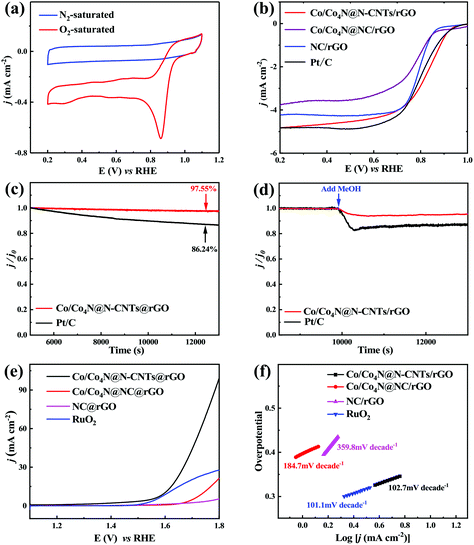
To clarify the relationship between the catalytic activity and the typical structure/composite of Co/Co4N@N-CNTs/rGO, the electrocatalytic performance of three ZIF-derived samples was also studied in the three-electrode system. Fig. 3a shows the cyclic voltammetry (CV) scan results of the Co/Co4N@N-CNTs/rGO catalyst in 0.1 M KOH solution with saturated O2 or N2. As shown in Fig. 3a, a typical oxygen reduction peak appeared at around ∼0.8 V vs. reversible hydrogen electrode (RHE) when the electrolyte was filled with O2 gas, but no peak was found for the N2-saturated electrolyte. This phenomenon indicates that Co/Co4N@N-CNTs/rGO has ORR activity in alkaline solution. In addition, the linear sweep voltammetry (LSV) profiles of the Co/Co4N@N-CNTs/rGO, Co/Co4N@NC/rGO, NC/rGO, and Pt/C catalysts (20 wt% platinum on Vulcan XC-72 carbon) were analyzed to better understand their ORR activities. It can be found from Fig. 3b that the limited current densities of the Co/Co4N@N-CNTs/rGO, Co/Co4N@NC/rGO, NC/rGO, and Pt/C catalysts are 4.82, 3.77, 4.23 and 4.82 mA cm−2, respectively. Co/Co4N@N-CNTs/rGO shows the lowest half-wave potential among the four catalysts (Fig. 3b). These results indicate that Co/Co4N@N-CNTs/rGO shows superior ORR catalytic activity, which may be due to the high (N-6 + N-Q) content and typical core–shell structure of Co/Co4N@N-CNTs/rGO. To further investigate the ORR kinetics activity of Co/Co4N@N-CNT@rGO, Koutecky–Levich (K–L) plots at different potentials were obtained through rotating disk electrode (RDE) tests (see the Experimental section in the ESI† for details). It can be found from Fig. S11† that all the K–L plots in the potential range of 0.2 to 0.5 V exhibit good linearity with similar slopes, which suggests first-order kinetics for the reaction on the Co/Co4N@N-CNT/rGO catalyst. In addition, the average electron transfer numbers of Co/Co4N@N-CNT/rGO were calculated to be ∼4.0 according to the K–L equation, indicating that the ORR process for the Co/Co4N@N-CNT/rGO catalyst is a typical four electron transfer pathway. Moreover, to detect the potential application of Co/Co4N@N-CNTs/rGO in metal–air batteries, the ORR durability of the Co/Co4N@N-CNTs/rGO and Pt/C catalysts was also investigated by chronoamperometric measurements in O2-saturated 0.1 M KOH solution. As shown in Fig. 3c, the normalized current of Co/Co4N@N-CNTs/rGO could retain 97.55% even after 8000 s, while Pt/C showed a relatively low current retention of 86.24%; this highlights the superior stability of the Co/Co4N@N-CNTs/rGO catalyst. Moreover, the resistance of Co/Co4N@N-CNTs/rGO and Pt/C to poisoning effects caused by methanol was assessed by chronoamperometric measurements in O2-saturated 0.1 M KOH with the addition of 5% volume methanol. The ORR current for Co/Co4N@N-CNTs/rGO showed no obvious variation after adding the methanol; however, the relative current of Pt/C exhibited a sharp decrease after the introduction of methanol, which suggests that the Co/Co4N@N-CNTs/rGO catalyst has highly stable ORR performance (Fig. 3d). On the other hand, the OER performance of the Co/Co4N@N-CNTs/rGO, Co/Co4N@NC/rGO, NC/rGO, and commercial RuO2 catalysts was also evaluated in KOH solution. As shown in Fig. 3e, Co/Co4N@N-CNTs/rGO displays an overpotential of 372.1 mV when the corresponding current density reaches 10 mA cm−2, which is much lower than NC/rGO (no result), Co/Co4N@rGO (511.8 mV) and RuO2 (371.9 mV). Furthermore, it can be observed from Fig. 3f that the corresponding Tafel slope of Co/Co4N@N-CNTs/rGO is smaller (102.7 mV per decade) compared with those of NC/rGO (359.8 mV per decade) and Co/Co4N@NC/rGO (184.7 mV per decade) and only slightly larger than that of RuO2 (101.1 mV per decade), suggesting the excellent OER performance of Co/Co4N@N-CNTs/rGO. Hence, Co/Co4N@N-CNTs/rGO reveals excellent catalytic activity towards ORR and OER; this may be due to the typical core–shell structure of Co/Co4N@N-CNTs, the existence of the rGO matrix, the high N content and the ultra-small Co/Co4N nanoparticles in Co/Co4N@N-CNTs/rGO.
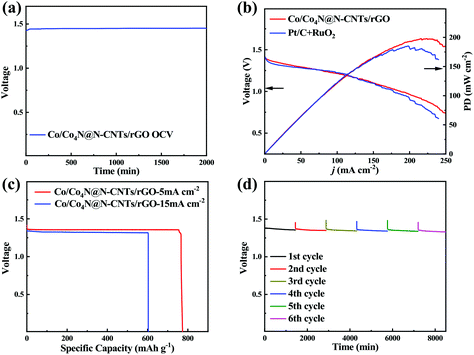
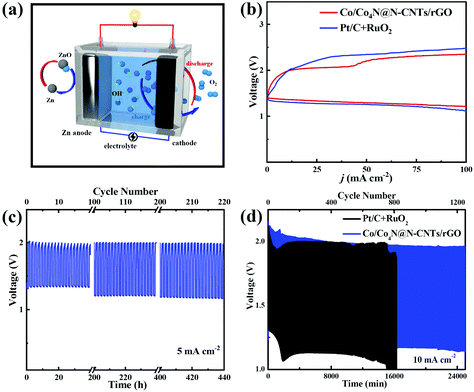
Encouraged by the excellent bifunctional ORR/OER catalytic activity of Co/Co4N@N-CNTs/rGO, a homemade Zn–air cell was assembled to assess its potential in energy devices. This Zn–air cell, which consists of the Zn anode, Co/Co4N@N-CNTs/rGO cathode and KOH electrolyte, was firstly investigated as the primary battery system. As shown in Fig. 4a, the open circuit voltage (OCV) of the Zn–air battery with the Co/Co4N@N-CNTs/rGO cathode can remain very stable above 1.45 V for 2000 min. In addition, Fig. 4b shows that the powder and discharge polarization curves of the Pt/C + RuO2 electrode are similar to those of the Co/Co4N@N-CNTs/rGO cathode; however, its peak power density (∼175 mW cm−2) is smaller than that of the Co/Co4N@N-CNTs/rGO-based Zn–air battery (∼200 mW cm−2), further indicating the excellent ORR activity of Co/Co4N@N-CNTs/rGO. A Zn–air battery with a Co/Co4N@N-CNTs/rGO cathode exhibits a high discharge capacity of 783 mA h gZn−1 (calculated based on the mass of consumed Zn metal) with a stable voltage platform of ∼1.34 V at 5 mA cm−2 (Fig. 4c); this is higher than those of most recently reported catalyst based Zn–air batteries (Table S1†). The discharge capacity of the Pt/C cathode at 5 mA cm−2 (Fig. S12†) is obviously smaller than those of the Co/Co4N@N-CNTs/rGO electrode. Even at a higher current of 15 mA cm−2, the discharge capacity of the Co/Co4N@N-CNTs/rGO electrode could still reach 603 mA h gZn−1, and its voltage platform slightly decreased to 1.31 V (Fig. 4c). These results demonstrate the excellent rate performance of the Co/Co4N@N-CNTs/rGO cathode. Moreover, the Zn–air battery with a Co/Co4N@N-CNTs/rGO cathode was mechanically recharged by replacing the Zn anode and KOH electrolyte every 24 h to assess its long-term discharge performance (Fig. 4d). As illustrated in Fig. 4d, the discharge voltage of the Zn–air battery with Co/Co4N@N-CNTs/rGO showed a very stable voltage platform above 1.33 V during six rounds of mechanical recharge at 5 mA cm−2, suggesting the outstanding ORR durability of the Co/Co4N@N-CNTs/rGO catalyst in Zn–air batteries. Furthermore, Fig. S13† shows that 16 parallel light-emitting diodes (LEDs) could be driven by two Co/Co4N@N-CNTs/rGO-based Zn–air batteries connected in series, suggesting its potential application. Moreover, we further studied the rechargeable performance of this Zn–air system with the Co/Co4N@N-CNTs/rGO cathode (Fig. 5a). In addition, the charge/discharge polarization curves of the Co/Co4N@N-CNTs/rGO and Pt/C + RuO2-based Zn–air batteries were investigated (Fig. 5b). It can be observed from Fig. 5b that the Co/Co4N@N-CNTs/rGO cathode exhibits smaller discharge/charge overpotentials than the Pt/C + RuO2 electrode at all current densities, which indicates that the Co/Co4N@N-CNTs/rGO-based Zn–air battery exhibits super-high ORR/OER activity. Moreover, galvanostatic discharge/charge measurements were applied to further evaluate the cycling performance of the Co/Co4N@N-CNTs/rGO cathode in a Zn–air battery. Fig. 5c shows the voltage curves of the Co/Co4N@N-CNTs/rGO-based Zn–air cell at a current density of 5 mA cm−2 with a limited time of 2 h per cycle. It can be found that the Zn–air battery using the Co/Co4N@N-CNTs/rGO catalyst could be stably cycled for 440 h with the stable charge/discharge voltage gap of ∼0.8 V, which is much better than most recently reported catalysts (Table S2†). When the fixed time was shortened to 20 min per cycle, the Zn–air battery with the Co/Co4N@N-CNTs/rGO cathode revealed almost no obvious voltage variation, with low overpotentials of ∼0.82 V over 1250 cycles at a current of 10 mA cm−2; meanwhile, the discharge/charge profiles of the Pt/C + RuO2-based Zn–air cell showed obviously increased voltage polarization after only 730 cycles. These results further demonstrate that the Co/Co4N@N-CNTs/rGO-based Zn–air batteries exhibit superior cycling stability with low overpotential due to the excellent ORR/OER catalytic activity and stability of Co/Co4N@N-CNTs/rGO.
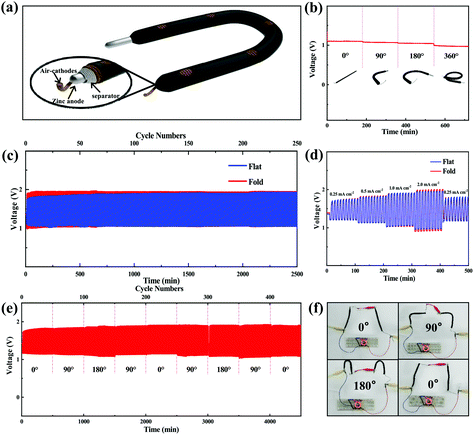
On the other hand, a flexible polymer-based Zn–air battery was also assembled using Co/Co4N@N-CNTs/rGO-coated carbon fiber rope as the air cathode, Zn wire as the anode and poly(vinyl alcohol) (PVA)-KOH hydrogel polymer as the electrolyte (Fig. 6a) to verify the potential application of Co/Co4N@N-CNTs/rGO in wearable devices. This polymer-based Zn–air cell with the Co/Co4N@N-CNTs/rGO cathode could maintain a relatively stable open circuit voltage (OCV) of ∼1.33 V under a series of bending conditions in sequence (Fig. S14†). Even when the flexible Co/Co4N@N-CNTs/rGO based Zn–air battery was discharged at the current density of 1 mA cm−2, the voltage curve of the cell was almost unchanged after orderly bending to 0° (flat), 90°, 180° and 360° (Fig. 6b). In addition, Fig. 6c shows the continuous discharge and charge profiles of the polymer-based Zn–air batteries using the Co/Co4N@N-CNTs/rGO catalyst under flat and folded conditions at 1 mA cm−2 with a limited time of 10 min per cycle. As shown in Fig. 6c, in both the flat and folded states, the flexible Co/Co4N@N-CNTs/rGO-based Zn–air batteries always revealed stable and similar charge/discharge voltage behavior over 250 cycles. The electrochemical performance of the flexible Co/Co4N@N-CNTs/rGO-based Zn–air cell at different current densities with 10 min per cycle under flat and folded states was also investigated (Fig. 6d). It can be observed from Fig. 6d that all the discharge/charge curves of the flexible Co/Co4N@N-CNTs/rGO-based Zn–air battery under flat or folded conditions are very stable, and the corresponding overpotentials only show a slight increase at currents varying from 0.25 to 2 mA cm−2. Especially, at 2 mA cm−2, the charge and discharge voltages of the flexible Co/Co4N@N-CNTs/rGO based Zn–air cell under flat conditions remained constant and were almost the same as those of the flexible battery in the folded state. Moreover, the charging and discharging potentials of the flexible Co/Co4N@N-CNTs/rGO based Zn–air battery at a current density of 0.5 mA cm−2 with a discharge/charge depth of 10 min per cycle were nearly invariable over 500 cycles, even under repeated bending to 0°, 90° and 180° in sequence (Fig. 6e). 16 parallel light-emitting diode (LED) lights were driven by two flexible Co/Co4N@N-CNTs/rGO-based Zn–air batteries connected in series, and the brightness of these LEDs remained almost the same even after bending to 0°, 90°, 180° and 0° (Fig. 6f). Interestingly, two flexible Zn–air batteries with a Co/Co4N@N-CNTs/rGO cathode could normally power these LEDs for 3 h, even under a bending angle of 180° (Fig. S15†). These results demonstrate that the Zn–air batteries using the Co/Co4N@N-CNTs/rGO cathode display high mechanical strength, excellent flexibility and good electrochemical performance; thus, they have potential application in the field of flexible power supplies.
In summary, ultra-small Co-based nanoparticles in situ encapsulated into N-CNTs-coated rGO nanosheets (Co/Co4N@N-CNTs/rGO) were successfully synthesized by pyrolysis of core–shell-structured ZIF-8@ZIF-67-modified GO (ZIF-8@ZIF-67/GO) and further applied as a bifunctional catalyst in Zn–air batteries. In situ encapsulation of Co/Co4N particles into N-CNTs can not only ensure their intimate contact to obviously improve the electronic conductivity of Co/Co4N@N-CNTs/rGO, but can also greatly inhibit the aggregation of Co-based nanoparticles over long-term cycling. Additionally, due to its numerous N-CNTs and uniformly distributed Co-based nanoparticles, Co/Co4N@N-CNTs/rGO exhibited excellent ORR and OER catalytic activity. With the introduction of rGO nanosheets and the elimination of Zn, the surface area and pores of Co/Co4N@N-CNTs/rGO could be significantly increased, which can effectively improve the reversible reactions in Zn–air batteries. Therefore, Co/Co4N@N-CNTs/rGO exhibits superior OER/ORR activity/stability, and the corresponding conventional Zn–air battery with a Co/Co4N@N-CNTs/rGO cathode revealed a higher power density (∼200 mW cm−2), longer cycling life (more than 440 cycles at 5 mA cm−2), lower discharge/charge overpotential (∼0.8 V) and larger discharge capacity (783 mA h gZn−1 at 5 mA cm−2) than cells using precious metal-based electrodes. More importantly, Co/Co4N@N-CNTs/rGO was also applied in a flexible polymer-based Zn–air cell, and the corresponding battery shows a high open circuit voltage (∼1.33 V), stable discharge profile and superior cycling durability (cycled for ∼5000 min at 0.5 mA cm−2) after a series of serious bending interferences; this suggests its potential application in powering wearable electrical devices. Overall, this initial proof of concept investigation not only opens new pathways to design metal-embedded carbon-based bifunctional catalysts for high-performance Zn–air cells, but also potentially promotes the development of flexible energy conversion systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding support from the National Natural Science Foundation of China (21905151 and 51772162), the Natural Science Foundation of Shandong Province (ZR2018BB034), the China Postdoctoral Science Foundation (2019M652499), Taishan Scholar Advantage and Characteristic Discipline Team of Eco Chemical Process and Technology, Guangxi Key Laboratory for Electrochemical Energy Materials, and the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry of Jilin University (2019–23).References
- Y. H. Zhu, S. Yuan, D. Bao, Y. B. Yin, H. X. Zhong, X. B. Zhang, J. M. Yan and Q. Jiang, Adv. Mater., 2017, 29, 1603719 CrossRef PubMed.
- J. Yi, P. C. Liang, X. Y. Liu, K. Wu, Y. Y. Liu, Y. G. Wang, Y. Y. Xia and J. J. Zhang, Energy Environ. Sci., 2018, 11, 3075–3095 RSC.
- H. X. Zhong, Q. Zhang, J. Wang, X. B. Zhang, X. L. Wei, Z. J. Wu, K. Li, F. L. Meng, D. Bao and J. M. Yan, ACS Catal., 2018, 8, 3965–3970 CrossRef CAS.
- X. Y. Yang, J. J. Xu, Z. W. Chang, D. Bao, Y. B. Yin, T. Liu, J. M. Yan, D. P. Liu, Y. Zhang and X. B. Zhang, Adv. Energy Mater., 2018, 8, 1702242 CrossRef.
- J. Pan, Y. Y. Xu, H. Yang, Z. Dong, H. Liu and B. Y. Xia, Adv. Sci., 2018, 5, 1700691 CrossRef PubMed.
- Z. D. Yang, X. Y. Yang, T. Liu, Z. W. Chang, Y. B. Yin, X. B. Zhang, J. M. Yan and Q. Jiang, Small, 2018, 14, 1800590 CrossRef PubMed.
- Y. Mao, G. Li, Y. Guo, Z. Li, C. Liang, X. Peng and Z. Lin, Nat. Commun., 2017, 8, 14628 CrossRef PubMed.
- Y. H. Zhu, X. Yang, D. Bao, X. F. Bie, T. Sun, S. Wang, Y. S. Jiang, X. B. Zhang, J. M. Yan and Q. Jiang, Joule, 2018, 2, 736–746 CrossRef CAS.
- R. P. Fang, K. Chen, L. C. Yin, Z. H. Sun, F. Li and H. M. Cheng, Adv. Mater., 2019, 31, 1800863 CrossRef PubMed.
- Y. Li and J. Lu, ACS Energy Lett., 2017, 2, 1370–1377 CrossRef CAS.
- H. X. Zhong, Y. Zhang and X. B. Zhang, Chem, 2018, 4, 196–198 CAS.
- Z. F. Huang, J. Wang, Y. Peng, C. Y. Jung, A. Fisher and X. Wang, Adv. Energy Mater., 2017, 7, 1700544 CrossRef.
- H. F. Wang, C. Tang and Q. Zhang, Adv. Funct. Mater., 2018, 28, 1803329 CrossRef.
- H. Yang, B. Wang, H. Li, B. Ni, K. Wang, Q. Zhang and X. Wang, Adv. Energy Mater., 2018, 8, 1801839 CrossRef.
- S. S. Shinde, C. H. Lee, A. Sami, D. H. Kim, S. U. Lee and J. H. Lee, ACS Nano, 2017, 11, 347–357 CrossRef CAS PubMed.
- P. Tan, B. Chen, H. Xu, H. Zhang, W. Cai, M. Ni, M. Liu and Z. Shao, Energy Environ. Sci., 2017, 10, 2056–2080 RSC.
- W. Wang, M. Tang, Z. Zheng and S. Chen, Adv. Energy Mater., 2019, 9, 1803628 CrossRef.
- Y. Wang, L. Tao, Z. Xiao, R. Chen, Z. Jiang and S. Wang, Adv. Funct. Mater., 2018, 28, 1705356 CrossRef.
- T. H. You and C. C. Hu, ACS Appl. Mater. Interfaces, 2018, 10, 10064–10075 CrossRef CAS PubMed.
- V. M. Dhavale and S. Kurungot, ACS Catal., 2015, 5, 1445–1452 CrossRef CAS.
- S. Hu, T. Han, C. Lin, W. Xiang, Y. Zhao, P. Gao, F. Du, X. Li and Y. Sun, Adv. Funct. Mater., 2017, 27, 1700041 CrossRef.
- Z. Guo, C. Li, W. Li, H. Guo, X. Su, P. He, Y. Wang and Y. Xia, J. Mater. Chem. A, 2016, 4, 6282–6289 RSC.
- W. P. Xiao, M. A. L. Cordeiro, G. Y. Gao, A. M. Zheng, J. Wang, W. Lei, M. X. Gong, R. Q. Lin, E. Stavitski, H. L. Xin and D. L. Wang, Nano Energy, 2018, 50, 70–78 CrossRef CAS.
- J. Liu, M. Jiao, L. Lu, H. M. Barkholtz, Y. Li, Y. Wang, L. Jiang, Z. Wu, D. J. Liu, L. Zhuang, C. Ma, J. Zeng, B. Zhang, D. Su, P. Song, W. Xing, W. Xu, Y. Wang, Z. Jiang and G. Sun, Nat. Commun., 2017, 8, 15938 CrossRef CAS PubMed.
- Y. Guo, P. Yuan, J. Zhang, H. Xia, F. Cheng, M. Zhou, J. Li, Y. Qiao, S. Mu and Q. Xu, Adv. Funct. Mater., 2018, 28, 1805641 CrossRef.
- Q. Hu, G. M. Li, G. D. Li, X. F. Liu, B. Zhu, X. Y. Chai, Q. L. Zhang, J. Liu and C. He, Adv. Energy Mater., 2019, 9, 1803867 CrossRef.
- H. Jiang, J. X. Gu, X. S. Zheng, M. Liu, X. Q. Qiu, L. B. Wang, W. Z. Li, Z. F. Chen, X. B. Ji and J. Li, Energy Environ. Sci., 2019, 12, 322–333 RSC.
- Z. Pei, H. Li, Y. Huang, Q. Xue, Y. Huang, M. Zhu, Z. Wang and C. Zhi, Energy Environ. Sci., 2017, 10, 742–749 RSC.
- J. Wang, W. Liu, G. Luo, Z. J. Li, C. Zhao, H. R. Zhang, M. Z. Zhu, Q. Xu, X. Q. Wang, C. M. Zhao, Y. T. Qu, Z. K. Yang, T. Yao, Y. F. Li, Y. Lin, Y. Wu and Y. D. Li, Energy Environ. Sci., 2018, 11, 3375–3379 RSC.
- L. Wang, Y. Q. Wang, M. G. Wu, Z. X. Wei, C. Y. Cui, M. L. Mao, J. T. Zhang, X. P. Han, Q. H. Liu and J. M. Ma, Small, 2018, 14, 1800737 CrossRef PubMed.
- H. Zhang, T. Wang, A. Sumboja, W. Zang, J. Xie, D. Gao, S. J. Pennycook, Z. Liu, C. Guan and J. Wang, Adv. Funct. Mater., 2018, 28, 1804846 CrossRef.
- C. Tang, B. Wang, H. F. Wang and Q. Zhang, Adv. Mater., 2017, 29, 1703185 CrossRef PubMed.
- H. F. Wang, C. Tang, B. Wang, B. Q. Li and Q. Zhang, Adv. Mater., 2017, 29, 1702327 CrossRef PubMed.
- L. Wei, H. E. Karahan, S. Zhai, H. Liu, X. Chen, Z. Zhou, Y. Lei, Z. Liu and Y. Chen, Adv. Mater., 2017, 29, 1701410 CrossRef PubMed.
- S. Li, C. Cheng, X. Zhao, J. Schmidt and A. Thomas, Angew. Chem., Int. Ed., 2018, 57, 1856–1862 CrossRef CAS PubMed.
- Z. Y. Guo, F. M. Wang, Y. Xia, J. L. Li, A. G. Tamirat, Y. R. Liu, L. Wang, Y. G. Wang and Y. Y. Xia, J. Mater. Chem. A, 2018, 6, 1443–1453 RSC.
- B. H. Chen, X. B. He, F. X. Yin, H. Wang, D. J. Liu, R. X. Shi, J. N. Chen and H. W. Yin, Adv. Funct. Mater., 2017, 27, 1700795 CrossRef.
- Q. Lu, J. Yu, X. H. Zou, K. M. Liao, P. Tan, W. Zhou, M. Ni and Z. P. Shao, Adv. Funct. Mater., 2019, 1904481 CrossRef.
- C. L. Li, M. C. Wu and R. Liu, Appl. Catal., B, 2019, 244, 150–158 CrossRef CAS.
- L. N. Liu, F. Yan, K. Y. Li, C. L. Zhu, Y. Xie, X. T. Zhang and Y. J. Chen, J. Mater. Chem. A, 2019, 7, 1083–1091 RSC.
- X. P. Han, X. F. Ling, Y. Wang, T. Y. Ma, C. Zhong, W. B. Hu and Y. D. Deng, Angew. Chem., Int. Ed., 2019, 58, 5359–5364 CrossRef CAS PubMed.
- W. Wei, X. M. Shi, P. Gao, S. S. Wang, W. Hu, X. X. Zhao, Y. M. Ni, X. Y. Xu, Y. Q. Xu, W. S. Yan, H. X. Ji and M. H. Cao, Nano Energy, 2018, 52, 29–37 CrossRef CAS.
- S. Liu, M. Wang, X. Sun, N. Xu, J. Liu, Y. Wang, T. Qian and C. Yan, Adv. Mater., 2018, 30, 1704898 CrossRef PubMed.
- F. Meng, H. Zhong, D. Bao, J. Yan and X. Zhang, J. Am. Chem. Soc., 2016, 138, 10226–10231 CrossRef CAS PubMed.
- L. L. Zou, C. C. Hou, Z. Liu, H. Pang and Q. Xu, J. Am. Chem. Soc., 2018, 140, 15393–15401 CrossRef CAS PubMed.
- D. X. Ji, L. Fan, L. L. Li, S. J. Peng, D. S. Yu, J. N. Song, S. Ramakrishna and S. J. Guo, Adv. Mater., 2019, 31, 1808267 CrossRef PubMed.
- Y. Hu, H. Yang, J. Chen, T. Xiong, M. J. T. Balogun and Y. Tong, ACS Appl. Mater. Interfaces, 2019, 11, 5152–5158 CrossRef CAS PubMed.
- Z. Y. Guo, F. M. Wang, Z. J. Li, Y. Yang, A. G. Tamirat, H. C. Qi, J. S. Han, W. Li, L. Wang and S. H. Feng, J. Mater. Chem. A, 2018, 6, 22096–22105 RSC.
- C. Hang, J. Zhang, J. W. Zhu, W. Q. Li, Z. K. Kou and Y. H. Huang, Adv. Energy Mater., 2018, 8, 1703539 CrossRef.
- M. J. Yang, X. H. Hu, Z. S. Fang, L. Sun, Z. K. Yuan, S. Y. Wang, W. Hong, X. D. Chen and D. S. Yu, Adv. Funct. Mater., 2017, 27, 1701971 CrossRef.
- L. Zhao, X. L. Sui, J. Z. Li, J. J. Zhang, L. M. Zhang, G. S. Huang and Z. B. Wang, Appl. Catal., B, 2018, 231, 224–233 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nr07270e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |