Scalable fabrication of high-performance micro-supercapacitors by embedding thick interdigital microelectrodes into microcavities†
Bangbang
Nie
a,
Xiangming
Li
*a,
Jinyou
Shao
 *a,
Congming
Li
a,
Pengcheng
Sun
b,
Yingche
Wang
c,
Hongmiao
Tian
a,
Chunhui
Wang
a and
Xiaoliang
Chen
a
*a,
Congming
Li
a,
Pengcheng
Sun
b,
Yingche
Wang
c,
Hongmiao
Tian
a,
Chunhui
Wang
a and
Xiaoliang
Chen
a
aMicro- and Nano-technology Research Center, State Key Laboratory for Manufacturing Systems Engineering, Xi'an Jiaotong University, Xi'an, Shaanxi 710049, China. E-mail: xiangmingli@xjtu.edu.cn; jyshao@mail.xjtu.edu.cn
bDepartment of Materials Science and Engineering, Materials Research Laboratory, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA
cXi'an Institute of Electromechanical Information Technology, Xi'an, Shaanxi 710065, China
First published on 5th August 2019
Abstract
Micro-supercapacitors (MSCs) with thick interdigital microelectrodes of carbon-based materials exhibit excellent electrochemical performance and hold tremendous promise for applications in microscale energy storage devices. Here, a scalable strategy to fabricate thick embedded multiwalled carbon nanotubes (MWCNTs) as interdigital microelectrodes for MSCs has been developed and investigated. To this end, sufficient MWNCT inks are firstly cast onto pre-patterned microcavity surfaces and then more MWCNT materials are embedded into the microcavities by rapid solvent evaporation. After removal of residual materials from the surfaces by a doctor-blading process, thick interdigital MWCNT microelectrodes with heights up to 190 μm are obtained. These embedded microelectrodes simplify the device structure and improve the mechanical flexibility by acting as both active materials and current collectors. Using interdigital microelectrodes with a width of 250 μm and an interspace of 50 μm, the fabricated MSCs exhibit outstanding electrochemical performance with a high capacitance of 19.5 mF cm−2 and an energy density of 2.48 μW h cm−2 at a power density of 24.7 μW cm−2. On the other hand, four light emitting diodes (LEDs) are successfully powered by three series of MSCs, indicating that MSCs can be connected in series and parallel to yield suitable operating voltages and currents for practical applications.
1. Introduction
The integration of microscale energy storage devices into electronic components, which generally are miniaturized, portable and wearable electronic devices, is highly required for the rapid development of the electronics industry.1–9 Although microbatteries are commercially manufactured as the main choice for micro-power systems, their disadvantages of low power density and short cycle life have greatly limited their widespread applications in micoelectronic devices, especially in implantable electronic medical devices.10–12 Therefore, tremendous efforts have been devoted to fabricate micro-energy storage devices to replace microbatteries.13 Among these, micro-supercapacitors (MSCs) are ideal alternatives to microbatteries due to their large storage capacities, long cycle life, and high power densities.14,15 Traditionally, microelectronic devices are powered by MSCs made of vertical sandwich structures but this can easily lead to short circuits of top and bottom electrodes.16 To prevent device failure caused by short circuits, sufficiently thick active materials must be filled between the two electrodes to ensure necessary distance separation. However, this would increase ion transport resistance, resulting in low power density devices. In addition, such components would be difficult to integrate into microelectronic devices due to their cumbersome sandwich structures. Compared to conventional MSCs made of sandwich structures, in-plane MSCs with interdigital electrodes are more suitable for integrated circuits.17–20Carbon-based materials with high electrical conductivities, outstanding mechanical strengths and large surface area-to-volume ratios, such as active carbon, carbon nanotubes (CNTs) and graphene, have been extensively researched for the fabrication of in-plane interdigital electrodes of MSCs.21–23 In these devices, the electrical energy is stored by surface charge separation at the interface between the electrode and electrolyte based on the electric double layer capacitor mechanism.24 For carbon-based interdigital microelectrodes of MSCs, great efforts have so far been made to develop all kinds of fabrication technologies, including photolithographic,25,26 inkjet and screen printing,27–30 and laser-induced methods.31–34 Photolithographic fabrication is a mature technology, which can be used to prepare interdigital carbon-based electrodes for MSCs. However, this route involves cumbersome procedures, such as spin-coating, ultraviolet (UV) exposure and development of photoresists, sputtering of metals, and plasma etching.25 These features limit the application of photolithography in the fabrication of efficient in-plane MSCs.35 In comparison, inkjet printing and screen printing are simpler and low-cost printing techniques, which might easily be used for the preparation of interdigital carbon-based electrodes for MSCs on various flexible substrates. However, inks with suitable viscosity cannot be easily prepared for all carbon-based materials, and the printing resolution often limits the electrochemical performance of MSCs.36 Besides the above methods, some researchers have developed other strategies to fabricate interdigital electrodes of MSCs based on carbon materials, such as multiwalled carbon nanotube (MWCNT) interdigital electrodes fabricated by injecting MWCNT inks into polydimethylsiloxane (PDMS) microcavities with hydrophilic treatment as well as graphene interdigital electrodes assembled by flowing graphene ink into microcavities under the action of capillary forces.37,38 However, thick interdigital electrodes are difficult to obtain only by improved wettability or capillary actions, leading to poor capacitance since only thin materials participate in the electrochemical reactions. To the best of our knowledge, it is challenging to fabricate thick interdigital electrodes for high-performance MSCs.39
In this work, we develop a scalable strategy to fabricate flexible in-plane and all-solid-state MSCs based on thick interdigital microelectrodes by using a doctor-blading process to embed MWCNTs into pre-patterned microcavities. Interdigital MWCNT microelectrodes with heights up to 190 μm can be obtained for high-performance MSCs. The thick embedded microelectrodes serve as both active materials and current collectors, simplifying the device structure and improving mechanical flexibility. The proposed strategy also solves problems related to fracturing or delamination caused by mechanical stress at the interface between active materials and metal current collectors. The fabricated MSCs exhibit outstanding electrochemical performance with a high capacitance of 19.5 mF cm−2 and an energy density of 2.48 μW h cm−2 at a power density of 24.7 μW cm−2. Moreover, their mechanical robustness is demonstrated under a bending radius of 7.5 mm and after 2000 bending cycles. On the other hand, the MSCs can be connected in series and parallel to obtain suitable operating voltages and currents to power four light emitting diodes (LEDs) with three MSC series. Overall, the proposed strategy to fabricate high-performance MSCs as energy storage devices for microelectronic devices is of low-cost and high efficiency.
2. Experimental section
2.1. Materials and equipment
MWCNT inks were commercially purchased from XFNANO (China) and dispersed in water at concentrations of 10 wt%. PVA was obtained from Aladdin and H3PO4 from TianJin ZhiYuan Reagent Co., Ltd. The PDMS prepolymer and cross-linker were purchased from Axson. The UV curable polymer was made of Norland Optical Adhesive 71 provided by LIENHE (China). The morphologies of interdigital microcavities and microelectrodes were visualized by confocal laser scanning microscopy (CLSM, OLS4000, Olympus) and scanning electron microscopy (SEM, SU8010, Hitachi). The electrochemical performance was evaluated using an electrochemical workstation (VersaSTAT3, Princeton Applied Research).2.2. Fabrication of interdigital microcavities
To obtain PDMS templates with raised interdigital structures, the PDMS prepolymer and cross-linker were first mixed at a weight ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and then stirred for 10 min. The mixture was cast onto Si molds with interdigital microcavity structures followed by degassing through vacuum assistance. The required PDMS templates were formed and then peeled off from the Si molds fabricated by conventional photolithography and dry etching processes. Next, the PDMS templates were used for imprinting the UV curable polymer dropped on polyethylene terephthalate (PET) films. The UV curable polymer was cured by exposure to UV light for 10 min. Finally, the interdigital microcavities of the UV curable polymer on PET films were obtained.
1 and then stirred for 10 min. The mixture was cast onto Si molds with interdigital microcavity structures followed by degassing through vacuum assistance. The required PDMS templates were formed and then peeled off from the Si molds fabricated by conventional photolithography and dry etching processes. Next, the PDMS templates were used for imprinting the UV curable polymer dropped on polyethylene terephthalate (PET) films. The UV curable polymer was cured by exposure to UV light for 10 min. Finally, the interdigital microcavities of the UV curable polymer on PET films were obtained.
2.3. Fabrication of interdigital MWCNT microelectrodes
First, the PET films with interdigital microcavities were treated with oxygen plasma (300 W, 50 s) until becoming hydrophilic. Next, sufficient amounts of MWCNT inks were cast onto pre-patterned interdigital microcavity surfaces of the PET films. A doctor blade was used to wade and drag the MWCNT inks forward–backward over the PET films. During the movement of the doctor blade, the dispersed MWCNTs filled the interdigital microcavities by a capillary force acting on the microcavities. Next, the MWCNT inks in the microcavities were dried on a hot plate for several min at 90 °C, and excess MWCNTs were removed from the PET films by ethanol-assisted scraping. After sintering at 130 °C for 30 min, interdigital MWCNT microelectrodes were obtained. The density of the MWCNT microelectrodes was about 850 mg cm−3.2.4. Preparation of the gel electrolyte
The mass fraction ratio of poly(vinyl alcohol)/phosphoric acid (PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H3PO4) in the polymer electrolyte was set to 1
H3PO4) in the polymer electrolyte was set to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8. First, PVA was slowly added into a certain amount of water under constant stirring. After PVA was stirred for 2 h at 95 °C, the solution became transparent. Next, after H3PO4 was slowly added into the PVA solution and stirred for 1 h, the gel electrolyte of PVA/H3PO4 was formed.
0.8. First, PVA was slowly added into a certain amount of water under constant stirring. After PVA was stirred for 2 h at 95 °C, the solution became transparent. Next, after H3PO4 was slowly added into the PVA solution and stirred for 1 h, the gel electrolyte of PVA/H3PO4 was formed.
2.5. Preparation of MSCs
Silver paste was used on both sides of interdigital MWCNT microelectrodes and copper tapes were attached to the silver paste to enable proper connection for testing. The gel electrolyte of PVA/H3PO4 was carefully cast on interdigital MWCNT microelectrodes followed by solidification for 24 h to yield flexible in-plane and all-solid-state MSCs.2.6. Electrochemical characterization and calculations
The electrochemical performance of the devices was evaluated by cyclic voltammetry (CV), galvanostatic charge/discharge (GCD), and electrochemical impedance spectroscopy (EIS) characterization, performed on the electrochemical workstation.The areal capacitance (C) was calculated from the CV curves and GCD curves according to the following equations:
 | (1) |
 | (2) |
The energy density and power density were calculated according to the following equations:
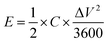 | (3) |
 | (4) |
3. Results and discussion
Fig. 1 shows a schematic diagram of the fabrication process of flexible in-plane and all-solid-state MSCs using thick embedded MWCNT interdigital microelectrodes by the doctor-blading process. Interdigital microcavities were first fabricated using PDMS templates made of raised interdigital structures to imprint UV curable polymers on PET films. The PDMS templates were prepared from Si molds with interdigital microcavities fabricated through traditional lithography and etching. As shown in Fig. 1a(i), sufficient MWCNT inks were cast on the microcavity surfaces, and more MWCNT solid content was filled into the microcavities after evaporation of the inks as shown in Fig. 1a(ii). After residual MWCNTs were wiped from the surfaces using anhydrous ethanol to prevent short circuits, MWCNT microelectrodes were obtained in the microcavities (Fig. 1a(iii)). Finally, the gel electrolyte of PVA/H3PO4 was cast onto the interdigital microelectrodes, and silver paste was applied on both sides of the interdigital microelectrodes to form MSCs (Fig. 1a(iv)). The electrochemical performance of MSCs is greatly affected by the structures, including the width, interspace and thickness, of interdigital microelectrodes.40 As shown in Fig. 1b, c and d, interdigital MWCNT microelectrodes with different widths (150 μm, 100 μm, and 250 μm) and interspaces (150 μm, 100 μm, and 50 μm) were obtained. The SEM image of the pre-patterned interdigital microcavities with a depth of 200 μm was obtained as shown in Fig. 1e. After the doctor-blading process, the MWCNTs were well embedded into the interdigital microcavities as shown in Fig. 1f. The porous structures of MWCNT microelectrodes are indicated by the enlarged image of the embedded MWCNTs in the inset of Fig. 1f. The density of the embedded MWCNT microelectrodes was about 850 mg cm−3 and the porous structures would improve ion transport for higher power capability. Moreover, thick interdigital MWCNT microelectrodes with high electrical conductivity and a large surface area can serve as active materials and current collectors without requiring additional deposition of metal current collectors. These features surely simplify the device structures. Furthermore, the absence of interfaces between the active materials and metal current collectors improves the mechanical flexibility of MSC devices by eliminating problems linked to fracturing or delamination caused by mechanical stress at MSC interfaces.41 Moreover, using the proposed process, a scalable fabrication of interdigital MWCNT microelectrodes can be successfully achieved at low cost with high efficiency (Fig. 1g).In the structures of the interdigital microelectrodes, the thickness of the microelectrodes is an important factor affecting the device performance of MSCs. For the accurate evaluation of the true performance of MSCs for practical applications, the areal capacitance instead of volumetric capacitance and gravimetric capacitance is used for assessing the capacitive performance.38 Hence, the areal capacitance was calculated using a geometric microelectrode area of 10 mm × 10 mm, including the entire projected surface areas of MWCNT microelectrodes and their interspaces. Fig. 2a shows the GCD curves of MSCs with different thicknesses of microelectrodes at a current density of 0.1 mA cm−2 at the same width and interspace of interdigital MWCNT microelectrodes. Fig. 2b shows the capacitance of MSCs at different thicknesses of MWCNT microelectrodes with the same width of 100 μm and interspace of 100 μm. MSCs with thicker MWCNT microelectrodes would exhibit better device performance due to the presence of more active materials involved in the electrochemical reactions. To the best of our knowledge, the fabrication of thick carbon-based electrodes for MSCs is still challenging. Kim et al. developed selective wetting-induced electrodes of MWCNTs for MSCs but a thickness of only around 5 μm was achieved with flowing MWCNT inks.37 The thickness of the CNT interdigital electrodes obtained by the 3-dimensional printing technique was 27.6 μm.39 All-solid-state MSCs assembled by the laser-assisted dry transfer technique of vertically aligned CNTs had interdigital electrodes with a thickness of around 50 μm.42 The thickness of the porous CNT interdigital electrodes obtained by chemical vapor deposition was around 80 μm.43 Using the proposed doctor-blading process coupled with an adjustment in the heating time, MWCNT inks could successfully fill the microcavities and embedded interdigital MWCNT microelectrodes of different depths could be fabricated, as shown in Fig. 2c and d. Using heating periods of 2 min to 3 min at 90 °C, MWCNT microelectrodes with heights up to 190 μm could be obtained in the microcavities. In some filling processes previously reported, the filling process by injecting MWCNT inks into PDMS microcavities with hydrophilic treatment could produce microelectrodes with a height of about 5 μm.37 Under the action of capillary forces, the filling of graphene ink into the microcavities could only produce thin microelectrodes with a height of 0.35 μm.38 Our MWCNT microelectrodes were much thicker than the reported carbon-based electrodes, leading to better electrochemical performance. Although the filling process for CNT-carbon composite microstructures could produce thick microelectrodes, the high temperature in the pyrolysis process would limit the application of the process in flexible substrates.44 In our doctor-blading process, after the PDMS templates were prepared from Si molds with interdigital microcavities fabricated through traditional lithography and etching, expensive equipment, such as mask aligners, sputtering coating machines, plasma etching equipment, etc., would not be needed in the next process. Moreover, cumbersome processes, such as spinning coating, exposure, development, etching, etc., also would not been involved in the process. Furthermore, as shown in Fig. 1g, scalable fabrication of interdigital microelectrodes was easily achieved. Therefore, the proposed doctor-blading process was a low-cost and high-efficiency manufacturing route for flexible in-plane and all-solid-state MWNCT-based MSCs without the use of expensive equipment and cumbersome processes.
Interdigital MWCNT microelectrodes with different widths and interspaces were fabricated to further investigate the electrochemical performance of MSCs. Fig. 3 shows the electrochemical performance of MSCs using different interdigital MWCNT microelectrodes when the ratio of the electrode width to the interspace was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As shown in Fig. 3a, embedded microelectrodes with width and interspace values of 150 μm and 150 μm; 100 μm and 100 μm; and 50 μm and 50 μm were fabricated to yield MSCs denoted as MSC-1, MSC-2 and MSC-3, respectively. In addition, all these MSCs had an active area of about 1 cm2. Fig. S1† shows the CV curves of the fabricated MSC-1, MSC-2 and MSC-3 at different scan rates from 2 to 100 mV s−1. At the same scan rate of 50 mV s−1, the CV curves of all three MSCs exhibited the typical charge–discharge behavior of electric double layer capacitors (Fig. 3b). Moreover, the near-ideal triangular GCD curves at the current density of 0.1 mA cm−2 confirmed the desirable capacitive behavior (Fig. 3c). The voltage window was also limited to 1 V for stable operation, and the GCD curves of all these MSCs at different current intensities from 0.05 to 2 mA cm−2 are shown in Fig. S2.† The CV and GCD curves indicated that MSC-3 exhibited better electrochemical performance than MSC-1 and MSC-2. In addition, the typical EIS for the MSCs at frequencies ranging from 1 MHz to 100 mHz is shown in Fig. 3d. The EIS curves exhibited a sharply rising straight line at low frequencies and equivalent series resistance (ESR) values of 208.5 Ω, 171.2 Ω and 87.2 Ω in the high frequency region, respectively. The result indicated that the simultaneous decrease in the width and interspace of the microelectrodes led to smaller ESRs, which could boost the usable discharge voltage window and improve the capacity of the MSCs through a small decrease in voltage at the beginning of discharge. The interspace between the MWCNT microelectrodes was formed by the UV curable polymer walls. Although the presence of polymer walls hindered direct ion transport between the microelectrodes, it played an important role in preventing short-circuits of microelectrodes. However, owing to the revealed porous structures in MWCTN microelectrodes as shown in Fig. 1f, the electrolyte ions could pass through the porous microelectrodes in the vertical direction and migrate over the polymer walls form one electrode to the counter electrode.45 The decrease in the interspace obviously restricted the pathway for ion transport from one electrode to the counter electrode. Simultaneously, the reduction in width also diminished the pathway for ion transport inside the electrodes.13,25 These features facilitated the complete use of active materials and improved the electrochemical performance. Therefore, MSC-3 exhibited higher capacitance than the other two MSCs, as shown in Fig. 3e. However, although a reduction in the size of the interdigital microelectrodes is beneficial for improving the electrochemical performance, further decrease in the interspace more likely causes short-circuits in microelectrodes.
1. As shown in Fig. 3a, embedded microelectrodes with width and interspace values of 150 μm and 150 μm; 100 μm and 100 μm; and 50 μm and 50 μm were fabricated to yield MSCs denoted as MSC-1, MSC-2 and MSC-3, respectively. In addition, all these MSCs had an active area of about 1 cm2. Fig. S1† shows the CV curves of the fabricated MSC-1, MSC-2 and MSC-3 at different scan rates from 2 to 100 mV s−1. At the same scan rate of 50 mV s−1, the CV curves of all three MSCs exhibited the typical charge–discharge behavior of electric double layer capacitors (Fig. 3b). Moreover, the near-ideal triangular GCD curves at the current density of 0.1 mA cm−2 confirmed the desirable capacitive behavior (Fig. 3c). The voltage window was also limited to 1 V for stable operation, and the GCD curves of all these MSCs at different current intensities from 0.05 to 2 mA cm−2 are shown in Fig. S2.† The CV and GCD curves indicated that MSC-3 exhibited better electrochemical performance than MSC-1 and MSC-2. In addition, the typical EIS for the MSCs at frequencies ranging from 1 MHz to 100 mHz is shown in Fig. 3d. The EIS curves exhibited a sharply rising straight line at low frequencies and equivalent series resistance (ESR) values of 208.5 Ω, 171.2 Ω and 87.2 Ω in the high frequency region, respectively. The result indicated that the simultaneous decrease in the width and interspace of the microelectrodes led to smaller ESRs, which could boost the usable discharge voltage window and improve the capacity of the MSCs through a small decrease in voltage at the beginning of discharge. The interspace between the MWCNT microelectrodes was formed by the UV curable polymer walls. Although the presence of polymer walls hindered direct ion transport between the microelectrodes, it played an important role in preventing short-circuits of microelectrodes. However, owing to the revealed porous structures in MWCTN microelectrodes as shown in Fig. 1f, the electrolyte ions could pass through the porous microelectrodes in the vertical direction and migrate over the polymer walls form one electrode to the counter electrode.45 The decrease in the interspace obviously restricted the pathway for ion transport from one electrode to the counter electrode. Simultaneously, the reduction in width also diminished the pathway for ion transport inside the electrodes.13,25 These features facilitated the complete use of active materials and improved the electrochemical performance. Therefore, MSC-3 exhibited higher capacitance than the other two MSCs, as shown in Fig. 3e. However, although a reduction in the size of the interdigital microelectrodes is beneficial for improving the electrochemical performance, further decrease in the interspace more likely causes short-circuits in microelectrodes.
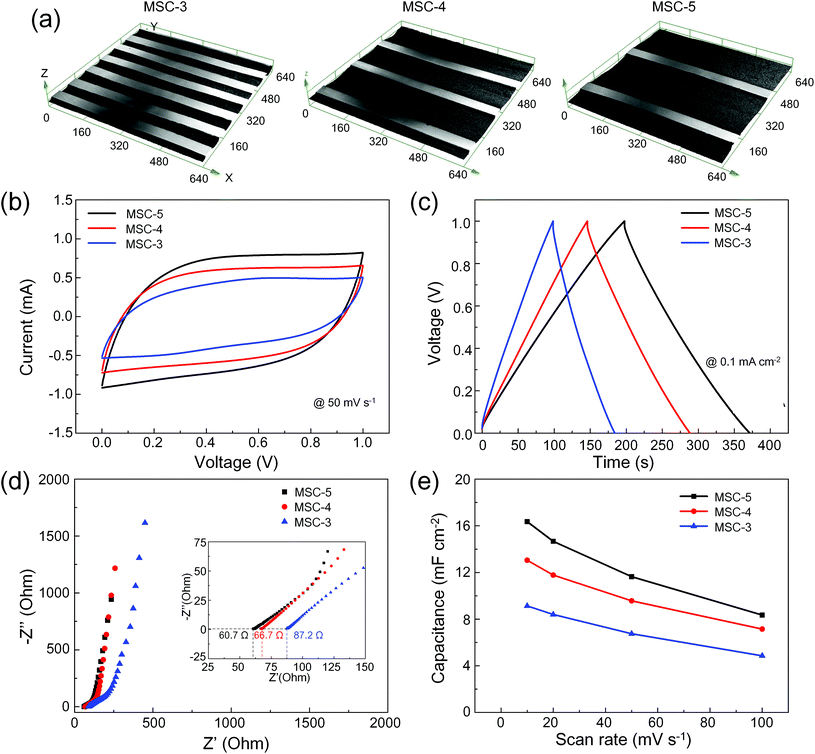
Fig. 4 shows the electrochemical performance of MSCs based on interdigital MWCNT microelectrodes with different widths at the same interspace of 50 μm. As shown in Fig. 4a, embedded microelectrodes with widths of 50 μm, 150 μm, and 250 μm were fabricated to yield MSCs denoted as MSC-3, MSC-4 and MSC-5, respectively. Fig. 4b shows the CV curves at a scan rate of 50 mV s−1 and Fig. 4c shows the GCD curves at a current density of 0.1 mA cm−2. The corresponding CV curves of these MSCs at different scan rates from 2 to 100 mV s−1 are shown in Fig. S3,† and the corresponding GCD curves at different current intensities from 0.05 to 2 mA cm−2 are shown in Fig. S4.† The CV and GCD curves indicated that MSC-5 exhibited better electrochemical performance than MSC-3 and MSC-4. EIS exhibited a sharply rising straight line at low frequencies with ESRs of 87.2 Ω, 66.7 Ω and 60.7 Ω at high frequencies for MSC-3, MSC-4 and MSC-5, respectively (Fig. 4d). Obviously, the increase in the width of the interdigital electrodes meant that more MWCNTs served as microelectrodes, hence boosting the electrical conductivity and reducing ESRs.14 Therefore, MSC-5 exhibited higher capacitance than the other two MSCs, as shown in Fig. 4e, due to more MWCNT materials being involved in electrochemical reactions. However, the approach was not appropriate for improving MSC performance as it arbitrarily increases the width of the electrodes.20 By moving the position from the edge to the electrode centers, the electric field became weaker and electrostatic forces driving the formation of the electric double layers reduced. Therefore, active materials near the electrode edges contributed more to electric double layer capacitance than those away from the edges. Increasing the electrode width not only decreased the ESRs, but also the electric field became uneven.36 Hence, the width and interspace of interdigital electrodes could be optimized to yield excellent electrochemical performance. Moreover, the design and fabrication of multilayer interdigital microelectrodes also would be beneficial for excellent electrochemical performance. The multilayer interdigital microelectrodes could be fabricated through two doctor-blading processes with two different inks. First, suitable conductive metal inks, such as silver ink and gold ink, can be firstly embedded into the bottom of the microcavities by the first doctor-blading process. In the first doctor-blading process, excess conductive inks will be removed from the PET films by ethanol-assisted scraping. Then, the metal ink will be sintered and can serve as metal current collectors. By an appropriate process, metal materials will be embedded into the bottom portion of the microcavities with sizes of only tens of microns. In the subsequent doctor-blading process, MWCNTs inks can be embedded into the microcavities and MWCNT materials will fill almost the rest of the microcavities. As shown Fig. S5,† in the multilayer interdigital microelectrodes, the Ag layers acted as metal current collectors and the MWCNT layers acted as active materials. At the same interspace of 100 μm and width of 100 μm, the MSCs with multilayer interdigital microelectrodes showed higher capacitance than the MWCNT interdigital microelectrodes. Therefore, the design and fabrication of multilayer interdigital microelectrodes would be important for pushing this field forward.
In our five fabricated MSCs with different structures, MSC-5 exhibited better electrochemical performance than the others. Fig. 5 shows excellent electrochemical and mechanical performance of MSC-5 devices. Near-ideal rectangular CV curves at different scan rates from 2 to 100 mV s−1 are shown in Fig. 5a and near-ideal triangular GCD curves at different current densities from 0.05 to 2 mA cm−2 are shown in Fig. 5b. As shown in Fig. S6,† the capacitance was changed by the scan rates and current densities, which indicated that different electrochemical measurements led to nearly the same capacitance values.38 At a scan rate of 2 mV s−1, the capacitance was estimated to be 19.5 mF cm−2, which was much higher than the most reported values of carbon-based MSCs. These values are 7.6 mF cm−2 for MSCs based on photochemically reduced graphene,35 0.08 mF cm−2 for MSCs based on methane plasma reduced graphene oxide (RGO),46 2.32 mF cm−2 for MSCs based on laser scribed RGO,31 3.93 mF cm−2 for MSCs based on graphene/carbon nanotube carpets (CNTCs),47 4.69 mF cm−2 for MSCs based on printing CNTs,39 and 5.63 mF cm−2 for MSCs based on RGO and black phosphorus quantum dot nanocomposites (BPQD)48 (details in Table 1). In addition to the excellent electrochemical performance, the fabricated MSCs showed good mechanical performance. To demonstrate the mechanical robustness of the MSCs, the device was attached on curved surfaces with different bending radii. Compared to the flat state, the CV curves almost retained the same shapes at 20 mV s−1 after bending of the device at different radii of 22.5 mm and 7.5 mm. The capacitance preserved 99.4% of the initial value even when the device was bent at a radius of 7.5 mm, demonstrating outstanding mechanical flexibility (Fig. 5c). Moreover, the fabricated MSCs maintained nearly 100% capacitance retention after 2000 cycles under a constant bending radius of 12.5 mm, showing outstanding mechanical stability (Fig. 5d). The embedded structures ensured that the MWCNT materials would not be destroyed by mechanical deformation, leading to good mechanical performance of the fabricated MSCs.
| Capacitance | Technique | Material | Ref. |
|---|---|---|---|
| 7.6 mF cm−2 | Photochemical reduction | RGO | 35 |
| 0.08 mF cm−2 | Methane plasma reduction | RGO | 46 |
| 2.32 mF cm−2 | Laser-scribed process | RGO | 31 |
| 3.93 mF cm−2 | Chemical vapor deposition | Graphene-CNTCs | 47 |
| 4.69 mF cm−2 | 3-Dimensional printing | CNTs | 39 |
| 5.63 mF cm−2 | Direct laser writing | RGO-BPQD | 48 |
| 19.5 mF cm−2 | Doctor-blading process | MWCNTs | This work |
To test the cycling stability, GCD measurements were performed at a current density of 1.5 mA cm−2. The capacitance did not decrease for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles, showing excellent cycling stability (Fig. 5e). The energy and power densities of MSC devices at different current densities from 0.05 to 2 mA cm−2 were calculated according to eqn (3) and (4). An energy density of 2.48 μW h cm−2 was obtained at a power density of 24.7 μW cm−2 and a power density of 638.3 μW cm−2 was recorded at an energy density of 0.555 μW h cm−2. Based on the electrode material, a volumetric energy density of 1.57 × 103 μW h cm−3 was obtained at a volumetric power density of 1.56 × 104 μW cm−3 and a volumetric power density of 4.03 × 105 μW cm−3 was recorded at a volumetric energy density of 3.51 × 102 μW h cm−3 for thick MWCNT microelectrodes. When the volumetric energy and power density are calculated based on the microdevices, the volume of the electrode material, UV polymer walls, the polymer electrolyte and PET substrates should be included for the microdevices. The volumetric energy density of 6.20 × 102 μW h cm−3 was obtained at a volumetric power density of 6.18 × 103 μW cm−3 and a volumetric power density of 1.60 × 105 μW cm−3 was recorded at a volumetric energy density of 1.39 × 102 μW h cm−3. The MSCs showed high areal energy and power densities due to more active materials involved in the electrochemical reaction in thick MWCNT microelectrodes. Energy and power densities were compared with different reported carbon-based MSCs as shown in Fig. 5f. The energy density of the MSCs based on the capillarity of graphene inks in interdigital microcavities was two orders of magnitude lower than that of our fabricated MSCs.38 The MSCs based on RGO/silver nanowire (AgNW) hybrid electrodes illustrated an energy density of 0.669 μW h cm−2 at a power density of 730 μW cm−2.5 MSCs based on graphene quantum dot interdigital electrodes showed an energy density of 0.154 μW h cm−2 at a power density of 7.51 μW cm−2.49 MSCs based on spray-coated MWCNT interdigital electrodes possessed an energy density of 6 μW h cm−2 at a power density of 0.34 μW cm−2.50 By comparison, our fabricated MSCs showed superior energy and power densities than most reported carbon-based MSCs.
000 charge–discharge cycles, showing excellent cycling stability (Fig. 5e). The energy and power densities of MSC devices at different current densities from 0.05 to 2 mA cm−2 were calculated according to eqn (3) and (4). An energy density of 2.48 μW h cm−2 was obtained at a power density of 24.7 μW cm−2 and a power density of 638.3 μW cm−2 was recorded at an energy density of 0.555 μW h cm−2. Based on the electrode material, a volumetric energy density of 1.57 × 103 μW h cm−3 was obtained at a volumetric power density of 1.56 × 104 μW cm−3 and a volumetric power density of 4.03 × 105 μW cm−3 was recorded at a volumetric energy density of 3.51 × 102 μW h cm−3 for thick MWCNT microelectrodes. When the volumetric energy and power density are calculated based on the microdevices, the volume of the electrode material, UV polymer walls, the polymer electrolyte and PET substrates should be included for the microdevices. The volumetric energy density of 6.20 × 102 μW h cm−3 was obtained at a volumetric power density of 6.18 × 103 μW cm−3 and a volumetric power density of 1.60 × 105 μW cm−3 was recorded at a volumetric energy density of 1.39 × 102 μW h cm−3. The MSCs showed high areal energy and power densities due to more active materials involved in the electrochemical reaction in thick MWCNT microelectrodes. Energy and power densities were compared with different reported carbon-based MSCs as shown in Fig. 5f. The energy density of the MSCs based on the capillarity of graphene inks in interdigital microcavities was two orders of magnitude lower than that of our fabricated MSCs.38 The MSCs based on RGO/silver nanowire (AgNW) hybrid electrodes illustrated an energy density of 0.669 μW h cm−2 at a power density of 730 μW cm−2.5 MSCs based on graphene quantum dot interdigital electrodes showed an energy density of 0.154 μW h cm−2 at a power density of 7.51 μW cm−2.49 MSCs based on spray-coated MWCNT interdigital electrodes possessed an energy density of 6 μW h cm−2 at a power density of 0.34 μW cm−2.50 By comparison, our fabricated MSCs showed superior energy and power densities than most reported carbon-based MSCs.
For practical use, the fabricated MSCs could be assembled in series and parallel configurations to obtain desirable output voltages and capacitances. The corresponding CV curves and GCD curves of two fabricated MSCs interconnected in series and parallel are presented in Fig. 6a and b. At the same scan rate of 100 mV s−1, the two MSCs in series exhibited an output voltage twice larger than that of a single MSC. Also, the voltage window was twice larger than that of a single MSC, without noticeable changes in the discharge time at the same current intensity of 1 mA cm−2. In addition, the two MSCs in parallel exhibited an output current twice that of a single MSC. On the other hand, the discharge time was also twice that of a single MSC, with the voltage window having almost the same area. As shown in Fig. 6c, three MSCs were designed in series to obtain a high voltage suitable for lighting up to four LEDs. Therefore, the fabricated MSCs would have potential applications as efficient microscale power sources for various devices requiring high operating currents and voltages.
4. Conclusions
In conclusion, the scalable fabrication of high-performance MSCs using embedded interdigital MWCNT microelectrodes using the doctor-blading process was demonstrated. The proposed strategy was suitable for the scalable fabrication of interdigital microelectrodes with low cost and high efficiency. Because of thick MWCNT microelectrodes up to 190 μm, the fabricated MSCs showed excellent electrochemical performance with a high capacitance of 19.5 mF cm−2. Moreover, they showed outstanding mechanical stability under different bending radii and bending times due to the embedded structures. Furthermore, the electrochemical performance was investigated for MSCs with different microelectrode structures, such as the thickness, width and interspace of the microelectrodes. The fabricated MSCs could easily be interconnected in series and parallel to obtain high operating voltages and currents for practical applications. Overall, the proposed strategy looks promising for the fabrication of MSCs as microscale energy storage devices in miniature electronics.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by National Key R&D Program of China (2017YFB1102900) and National Natural Science Foundation of China (Grant Numbers: 91323303).References
- S. Xu, W. Liu, B. Hu and X. Wang, Nano Energy, 2019, 58, 803–810 CrossRef CAS.
- L. Tong, M. Gao, C. Jiang and K. Cai, J. Mater. Chem. A, 2019, 7, 10751–10760 RSC.
- C. Lethien, J. L. Bideau and T. Brousse, Energy Environ. Sci., 2019, 12, 96–115 RSC.
- B. Nie, X. Li, J. Shao, X. Li, H. Tian, D. Wang, Q. Zhang and B. Lu, ACS Appl. Mater. Interfaces, 2017, 9, 40681–40689 CrossRef CAS PubMed.
- G.-W. Huang, N. Li, Y. Du, Q.-P. Feng, H.-M. Xiao, X.-H. Wu and S.-Y. Fu, ACS Appl. Mater. Interfaces, 2018, 10, 723–732 CrossRef CAS PubMed.
- J. Gao, C. Shao, S. Shao, F. Wan, C. Gao, Y. Zhao, L. Jiang and L. Qu, Small, 2018, 14, 1801809 CrossRef PubMed.
- H. Zhang, Y. Cao, M. O. L. Chee, P. Dong, M. Ye and J. Shen, Nanoscale, 2019, 11, 5807–5821 RSC.
- J. Liang, C. Jiang and W. Wu, Nanoscale, 2019, 11, 7041–7061 RSC.
- X. Li, J. Shao, S.-K. Kim, C. Yao, J. Wang, Y.-R. Miao, Q. Zheng, P. Sun, R. Zhang and P.-V. Braun, Nat. Commun., 2018, 9, 2578 CrossRef PubMed.
- N. Liu and Y. Gao, Small, 2017, 13, 1701989 CrossRef PubMed.
- N. A. Kyeremateng, T. Brousse and D. Pech, Nat. Nanotechnol., 2017, 12, 7–15 CrossRef CAS PubMed.
- A. Balducci, D. Belanger, T. Brousse, J. W. Long and W. Sugimoto, J. Electrochem. Soc., 2017, 164, 1487–A1488 CrossRef.
- H. Hu, Z. Pei and C. Ye, Energy Storage Mater., 2015, 1, 82–102 CrossRef.
- T. Lv, M. Liu, D. Zhu, L. Gan and T. Chen, Adv. Mater., 2018, 30, 1705489 CrossRef PubMed.
- S. Wang, Y. Yu, R. Li, G. Feng, Z. Wu, G. Compagnini, A. Gulino, Z. Feng and A. Hu, Electrochim. Acta, 2017, 241, 153–161 CrossRef CAS.
- Y.-Z. Zhang, Y. Wang, T. Cheng, L.-Q. Yao, X. Li, W.-Y. Lai and W. Huang, Chem. Soc. Rev., 2019, 48, 3229–3264 RSC.
- J. Zhao, Q. Shi, Y. Guo, X. Wang, D. Wang, F. Tan, L. Jiang and Y. Yu, J. Power Sources, 2019, 433, 226703 CrossRef CAS.
- L. Liu, Y. Feng, J. Liang, S. Li, B. Tian, W. Yao and W. Wu, J. Power Sources, 2019, 425, 195–203 CrossRef CAS.
- J. Du, Y. Zhao, Z. Zhang, X. Mu, X. Jiang, B. Huang, Y. Zhang, S. Zhang, Z. Zhang and E. Xie, J. Mater. Chem. A, 2019, 7, 6220–6227 RSC.
- D. Qi, Y. Liu, Z. Liu, L. Zhang and X. Chen, Adv. Mater., 2017, 29, 1602802 CrossRef PubMed.
- X. Zhang, Q. Fan, N. Qu, H. Yang, M. Wang, A. Liu and J. Yang, Nanoscale, 2019, 11, 8588–8596 RSC.
- Q. Xu, W. Li, L. Ding, W. Yang, H. Xiao and W.-J. Ong, Nanoscale, 2019, 11, 1475–1504 RSC.
- R. Zhang, J. Ding, C. Liu and E.-H. Yang, ACS Appl. Mater. Interfaces, 2018, 1, 2048–2055 CAS.
- Z. Zhao and Y. Xie, J. Power Sources, 2018, 400, 264–276 CrossRef CAS.
- Z.-S. Wu, K. Parvez, X. Feng and K. Müllen, J. Mater. Chem. A, 2014, 2, 8288–8293 RSC.
- S. Wang, B. Hsia, C. Carraro and R. Maboudian, J. Mater. Chem. A, 2014, 2, 7997–8002 RSC.
- H. Li and J. Liang, Adv. Mater., 2019, 1805864 CrossRef PubMed.
- S. Bellani, E. Petroni, A. E. Del Rio Castillo, N. Curreli, B. Martín-García, R. Oropesa-Nuñez, M. Prato and F. Bonaccorso, Adv. Funct. Mater., 2019, 29, 1807659 CrossRef.
- S. Wang, N. Liu, J. Tao, C. Yang, W. Liu, Y. Shi, Y. Wang, J. Su, L. Li and Y. Gao, J. Mater. Chem. A, 2015, 3, 2407–2413 RSC.
- K.-H. Choi, J. Yoo, C. K. Lee and S.-Y. Lee, Energy Environ. Sci., 2016, 9, 2812–2821 RSC.
- M. F. El-Kady and R. B. Kaner, Nat. Commun., 2013, 4, 1475 CrossRef PubMed.
- J. Lin, Z. Peng, Y. Liu, F. Ruiz-Zepeda, R. Ye, E. L. G. Samuel, M. J. Yacaman, B. I. Yakobson and J. M. Tour, Nat. Commun., 2014, 5, 5714 CrossRef CAS PubMed.
- H.-C. Huang, C.-J. Chung, C.-T. Hsieh, P.-L. Kuo and H. Teng, Nano Energy, 2016, 21, 90–105 CrossRef CAS.
- W. Song, J. Zhu, B. Gan, S. Zhao, H. Wang, C. Li and J. Wang, Small, 2018, 14, 1702249 CrossRef PubMed.
- S. Wang, Z.-S. Wu, S. Zheng, F. Zhou, C. Sun, H.-M. Cheng and X. Bao, ACS Nano, 2017, 11, 4283–4291 CrossRef CAS PubMed.
- B. Chen, Y. Jiang, X. Tang, Y. Pan and S. Hu, ACS Appl. Mater. Interfaces, 2017, 9, 28433–28440 CrossRef CAS PubMed.
- S.-K. Kim, H.-J. Koo, A. Lee and P. V. Braun, Adv. Mater., 2014, 26, 5108–5112 CrossRef CAS PubMed.
- W. J. Hyun, E. B. Secor, C.-H. Kim, M. C. Hersam, L. F. Francis and C. D. Frisbie, Adv. Energy Mater., 2017, 7, 1700285 CrossRef.
- W. Yu, H. Zhou, B. Q. Li and S. Ding, ACS Appl. Mater. Interfaces, 2017, 9, 4597–4604 CrossRef CAS PubMed.
- L. Liu, Z. Niu and J. Chen, Nano Res., 2017, 10, 1524–1544 CrossRef.
- L. Li, E. B. Secor, K.-S. Chen, J. Zhu, X. Liu, T. Z. Gao, J.-W. T. Seo, Y. Zhao and M. C. Hersam, Adv. Energy Mater., 2016, 6, 1600909 CrossRef.
- B. Hsia, J. Marschewski, S. Wang, J. B. In, C. Carraro, D. Poulikakos, C. P. Grigoropoulos and R. Maboudian, Nanotechnology, 2014, 25, 055401 CrossRef PubMed.
- Y. Q. Jiang, Q. Zhou and L. Lin, Proc. IEEE Micr. Elect., 2009, 587–590 Search PubMed.
- L. He, M. Toda, Y. Kawai, H. Miyashita, M. Omori, T. Hashida, R. Berger and T. Ono, Microsyst. Technol., 2014, 20, 201–208 CrossRef CAS.
- Y. Chen, M. Guo, L. He, W. Yang, L. Xu, J. Meng, X. Tian, X. Ma, Q. Yu, K. Yang, X. Hong and L. Mai, J. Materiomics, 2019, 5, 303–312 CrossRef.
- Z.-S. Wu, K. Parvez, X. Feng and K. Mullen, Nat. Commun., 2013, 4, 2487 CrossRef PubMed.
- J. Lin, C. Zhang, Z. Yan, Y. Zhu, Z. Peng, R. H. Hauge, D. Natelson and J. M. Tour, Nano Lett., 2013, 13, 72–78 CrossRef CAS PubMed.
- X.-Y. Fu, Z.-D. Chen, Y.-L. Zhang, D.-D. Han, J.-N. Ma, W. Wang, Z.-R. Zhang, H. Xia and H. B. Sun, Nanoscale, 2019, 11, 9133–9140 RSC.
- W.-W. Liu, Y.-Q. Feng, X.-B. Yan, J.-T. Chen and Q.-J. Xue, Adv. Funct. Mater., 2013, 23, 4111–4122 CrossRef CAS.
- H. Kim, J. Yoon, G. Lee, S.-H. Paik, G. Choi, D. Kim, B.-M. Kim, G. Zi and J. S. Ha, ACS Appl. Mater. Interfaces, 2016, 8, 16016–16025 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nr05247j |
| This journal is © The Royal Society of Chemistry 2019 |