Inception of molybdate as a “pore forming additive” to enhance the bifunctional electrocatalytic activity of nickel and cobalt based mixed hydroxides for overall water splitting†
Abstract
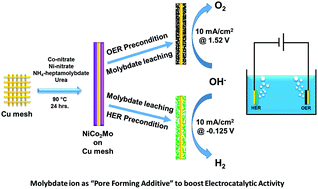
Development of low-cost transition metal based electrocatalysts on inexpensive substrates for overall water splitting is essential to meet the future energy storage demand. In this article, we have synthesized a molybdate incorporated nickel cobalt hydroxide material on Cu mesh with nickel : cobalt : molybdenum in a 13.25 : 21.42 : 1 ratio and the electrode has shown excellent bifunctional electrocatalytic activity as it demonstrates overpotentials as low as 290 mV and 125 mV to reach 10 mA cm−2geo for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), respectively (after both iR and capacitance correction). Control studies with fourteen other nickel–cobalt based hydroxides and rigorous post-catalytic analysis suggested that though molybdate was not the active catalytic centre, it played a pivotal role in enhancing the activity of the material as – (i) it significantly improved the surface area and porosity of the as-synthesized material and (ii) owing to its continuous etching during electrochemical testing, it was found to increase the accessibility of electrochemically active catalytic sites lying in the bulk. Thus, molybdate acts as a “pore forming additive” during both synthesis and electrochemical treatment. Furthermore, the combination of nickel and molybdate helped in the formation of a 2D-sheet like morphology which in turn improves accessibility to catalytically active centres. In addition, the Cu mesh substrate notably lowers the charge transfer resistance. To the best of our knowledge, this is the first ever report of molybdate as a “pore forming additive” and will enthuse the designing of electrocatalytic materials with enhanced performance based on this strategy.


 Please wait while we load your content...
Please wait while we load your content...