Magneto-mechanical actuation of magnetic responsive fibrous scaffolds boosts tenogenesis of human adipose stem cells†
Abstract
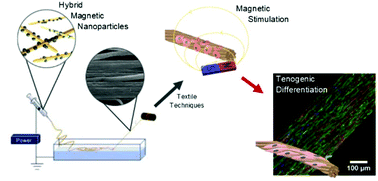
Tendons are highly specialized load-bearing tissues with very limited healing capacity. Given their mechanosensitive nature, the combination of tendon mimetic scaffolds with remote mechanical actuation could synergistically contribute to the fabrication of improved tissue engineered alternatives for the functional regeneration of tendons. Here, hybrids of cellulose nanocrystals decorated with magnetic nanoparticles were produced to simultaneously reinforce and confer magnetic responsiveness to tendon mimetic hierarchical fibrous scaffolds, resulting in a system that enables remote stimulation of cells in vitro and, potentially, in vivo after construct transplantation. The biological performance and functionality of these scaffolds were evaluated using human adipose stem cells (hASCs) cultured under or in the absence of magnetic actuation. It was demonstrated that magneto-mechanical stimulation of hASCs promotes higher degrees of cell cytoskeleton anisotropic organization and steers the mechanosensitive YAP/TAZ signaling pathway. As feedback, stimulated cells show increased expression of tendon-related markers, as well as a pro-healing profile in genes related to their inflammatory secretome. Overall, these results support the use of the proposed magnetic responsive fibrous scaffolds as remote biointegrated actuators that can synergistically boost hASC tenogenesis through mechanosensing mechanisms and may modulate their pro-healing paracrine signaling, thus collectively contributing to the improvement of the regenerative potential of engineered tendon grafts.
- This article is part of the themed collection: Editor’s Choice: Controlling anisotropy in nanomaterials


 Please wait while we load your content...
Please wait while we load your content...