Pattern formation of metal–oxide hybrid nanostructures via the self-assembly of di-block copolymer blends†
Abstract
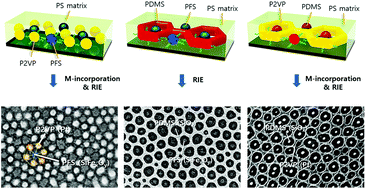
The templated self-assembly of block copolymers (BCPs) with a high Flory–Huggins interaction parameter (χ) can effectively create ultrafine, well-ordered nanostructures in the range of 5–30 nm. However, the self-assembled BCP patterns remain limited to possible morphological geometries and materials. Here, we introduce a novel and useful self-assembly method of di-BCP blends capable of generating diverse hybrid nanostructures consisting of oxide and metal materials through the rapid microphase separation of A–B/B–C BCP blends. We successfully obtained various hybridized BCP morphologies which cannot be acquired from a single di-BCP, such as hexagonally arranged hybrid dot and dot-in-hole patterns by controlling the mixing ratios of the solvents with a binary solvent annealing process. Furthermore, we demonstrate how the binary solvent vapor annealing process can provide a wide range of pattern geometries to di-BCP blends, showing a well-defined spontaneous one-to-one accommodation in dot-in-hole nanostructures. Specifically, we show clearly how the self-assembled BCPs can be functionalized via selective reduction and/or an oxidation process, resulting in the excellent positioning of confined silica nanodots into each nanospace of a Pt mesh. These results suggest a new method to achieve the pattern formation of more diverse and complex hybrid nanostructures using various blended BCPs.


 Please wait while we load your content...
Please wait while we load your content...