Measurements of aptamer–protein binding kinetics using graphene field-effect transistors†
Abstract
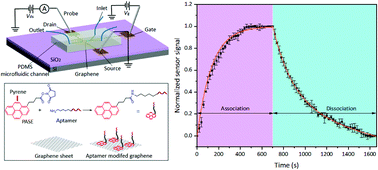
Quantifying interactions between biomolecules subject to various environmental conditions is essential for applications such as drug discovery and precision medicine. This paper presents an investigation of the kinetics of environmentally dependent biomolecular binding using an electrolyte-gated graphene field-effect transistor (GFET) nanosensor. In this approach, biomolecular binding occurring on and in the vicinity of a graphene surface induces a change in carrier concentration, whose resulting conductance change is measured. This allows a systematic study of the kinetic properties of the binding system. We apply this approach to the specific binding of human immunoglobulin E (IgE), an antibody involved in parasite immunity, with an aptamer at different ionic strengths (Na+ and Mg2+) and temperatures. Experimental results demonstrate increased-rate binding kinetics at higher salt-ion concentrations and temperatures. In particular, the divalent cation Mg2+ yields more pronounced changes in the conformational structure of the aptamer than the monovalent cation Na+. In addition, the dissociation of the aptamer-protein complex at room temperature is found to be characterized by large unfavorable changes in the activation enthalpy and entropy.


 Please wait while we load your content...
Please wait while we load your content...