Glucose regulation by modified boronic acid-sulfobetaine zwitterionic nanogels – a non-hormonal strategy for the potential treatment of hyperglycemia†
Abstract
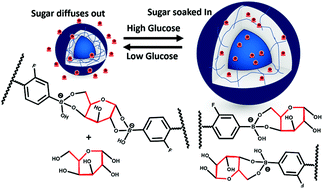
We have introduced a non-hormonal hyperglycemia treatment strategy by using an injectable glucose-responsive boronic acid- zwitterionic nanogel. The synthesized system, similar to an artificial liver, is capable of storing/releasing glucose at high/low blood glucose concentrations. In vivo performance revealed that the injection of the nanogels can effectively regulate blood glucose in type 1 diabetic rats for at least 6 hours.
- This article is part of the themed collection: 2019 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...