High piezo-resistive performances of anisotropic composites realized by embedding rGO-based chitosan aerogels into open cell polyurethane foams†
Abstract
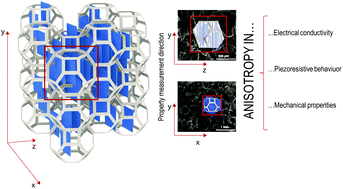
Anisotropic aerogel-foam composites were developed by embedding a reduced graphene oxide (rGO)/chitosan aerogel directly into an open-cell polyurethane foam through an in situ bidirectional freeze-drying process. The resulting aerogel–foam composites possess both excellent compression-resilience performance and stable piezo-resistive properties due, respectively, to the excellent mechanical properties of polyurethane foams and to the presence of a chitosan-based aerogel loaded with rGO. The latter, indeed, provides outstanding electrical properties due to its conductive and parallel flat lamellar structure. It has been proven that both mechanical and piezo-resistive properties are stable even after 1000 loading/unloading cycles and a reduction of the electrical resistance of about 86% is observed upon the application of a 60% strain. The high sensitivity, long cycling life, and reliable performance over a wide strain range make this unique anisotropic aerogel-foam composite a highly promising candidate for the production of wearable sensors and healthcare monitoring devices.
- This article is part of the themed collection: Editor’s Choice: Controlling anisotropy in nanomaterials


 Please wait while we load your content...
Please wait while we load your content...