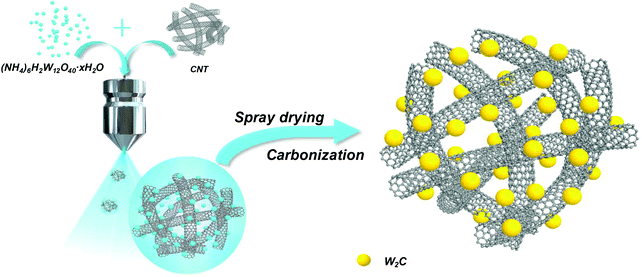
W2C nanodot-decorated CNT networks as a highly efficient and stable electrocatalyst for hydrogen evolution in acidic and alkaline media†
Yang
Hu
 ,
Bo
Yu
,
Bo
Yu
 ,
Wenxin
Li
,
Wenxin
Li
 ,
Manigandan
Ramadoss
,
Manigandan
Ramadoss
 and
Yuanfu
Chen
and
Yuanfu
Chen
 *
*
School of Electronic Science and Engineering, and State Key Laboratory of Electronic Thin Films and Integrated Devices, University of Electronic Science and Technology of China, Chengdu 610054, PR China. E-mail: yfchen@uestc.edu.cn; Tel: +86 28 83202710
First published on 12th February 2019
Abstract
Although tungsten carbide (W2C) has long been reported as an excellent platinum-like catalyst, it is still a challenge to synthesize W2C as an electrocatalyst for a highly efficient hydrogen evolution reaction (HER) due to its high onset overpotential, inevitable aggregation, and lack of a scalable and controllable synthesis method. Herein, we synthesized W2C nanodot-decorated CNT networks (W2C@CNT-S) via a facile and scalable spray drying method followed by a carbonization process. It is demonstrated that this unique nanoarchitecture, constructed by ultrafine W2C nanodots homogeneously decorated on a three-dimensional and conductive CNT skeleton, leads to the exposure of abundant catalytic sites and promotes highly efficient electron transfer and ion diffusion during the HER process. As a result, in acidic and alkaline media, the optimized W2C@CNT-S hybrid exhibited excellent HER performance with very low onset overpotentials of only 60 and 40 mV (vs. RHE) and very small Tafel slopes of 57.4 and 56.2 mV dec−1, and only needed 176 and 148 mV (vs. RHE) to obtain a current density of 10 mA cm−2, respectively; it also showed outstanding long-term durability even after a 30-hour test in both acidic and alkaline media. This study presents an overview of a low-cost and scalable spray-drying strategy to synthesize a high-performance carbide-based electrocatalyst for hydrogen evolution.
1. Introduction
Hydrogen has been considered to be a sustainable and clean alternative energy source to replace traditional fossil fuels in recent years. The production of hydrogen via electrocatalytic water splitting is safe and environmentally friendly when compared with the other hydrogen generation methods, including the steam reforming of hydrocarbons, the gasification reaction, and the partial oxidation reaction of heavy oil.1–4 Moreover, the most important reaction in water splitting is the hydrogen evolution reaction (HER), which has been drawing significant attention from researchers due to the accessible reactant and promise for large-scale production. Pt-Based catalysts have been reported as the most efficient electrocatalysts for HER; however, the high cost and scarcity of Pt seriously hinder the large-scale application of these catalysts for H2 production.5 Therefore, there is an urgent need to develop non-precious transition-metal-based electrocatalysts with low-cost, large-scale, efficient HER activity and excellent long-term stability.To date, non-precious transition-metal-based compounds, such as sulfides,6–8 selenides,9–12 phosphides,13,14 nitrides,15,16 and carbides,17–22 have been intensively investigated to replace the Pt-based electrocatalysts towards the HER. However, there are few electrocatalysts that can simultaneously satisfy the requirements of high electrocatalytic activity and excellent long-term durability, particularly in an alkaline medium. Among these catalysts, tungsten carbides (WC and W2C) have been proved to be a promising alternative to platinum for the HER due to their Pt-like d-band electronic density of states at the Fermi level.23 Some efforts have been made with W2C; however, W2C has received far less attention than the other tungsten carbide compounds (WC) for the HER because W2C is usually investigated as a by-product of WC. However, recent reports indicate that, compared to WC, W2C shows better HER performance.22–24 For example, Li's group synthesized W2C@NWs and WC@NWs via a two-step annealing treatment; compared to WC@NWs with a Tafel slope of 71 mV dec−1 in 0.5 M H2SO4, W2C@NWs delivered a smaller Tafel slope of 68 mV dec−1.24 It was noted that the formation of W2C required high temperature conditions over 1250 °C from the W–C phase diagram;25 however, at such high temperature, serious agglomeration is inevitable and it is not easy to obtain ideal nanoarchitecture with high electrocatalytic activity. So, it is still challenging to develop a scalable synthesis method to synthesize highly efficient and stable W2C for HER with a well-designed nanoarchitecture and less agglomeration. Although the overall thermal efficiency is low and it is not suitable for highly viscous materials, the spray-drying method has an advantage of realizing large-scale commercial production. Fortunately, Wei et al. and Yu et al. recently reported the successful synthesis of high-quality molybdenum carbide by a spray-drying process using graphene and CNTs as the carbon source and conductive framework, respectively.26,27 The spray-drying strategy might also be effective to suppress the agglomeration of W2C. However, to the best of our knowledge, there is no report on the synthesis of W2C with less agglomeration by spray drying, not to mention further control of the unique nanoarchitecture and enhancement of the HER performance of W2C in both acid and alkaline electrolytes.
To address these issues, herein, for the first time, we present a scalable and low-cost method to synthesize ultrafine W2C nanodots-decorated CNT networks via a spray-drying approach (designated as W2C@CNT-S) and a subsequent carbonization process. The synthesis process of W2C@CNT-S is schematically demonstrated in Fig. 1. Due to the chemical inertness of carbon nanotube, C atom diffusion through the solid–solid interface into the tungsten lattice becomes slow and effectively limits the overgrowth of W2C particles.23 Moreover, the unique structure with porous CNT networks as a carbon conductivity framework can facilitate the electron transfer; it is quite obvious that the ultrafine W2C nanodots homogenously decorated on highly conductive CNT guarantee the high conductivity and provide abundant reactive catalytic sites for the HER process. As expected, compared to the sample with W2C particles directly loaded on CNT without spray drying (designated as W2C/CNT), the optimized W2C@CNT-S hybrid exhibited a more efficient HER performance with lower onset overpotentials of ∼60 and 40 mV (vs. RHE), smaller Tafel slopes of 57.4 and 56.2 mV dec−1, and better stability, even after 30 h test in acidic and alkaline media, respectively.
2. Experimental
2.1. Synthesis of W2C@CNT-S and W2C/CNT
The W2C@CNT-S hybrid was synthesized as follows: 1.478 g of ammonium metatungstate hydrate ((NH4)6H2W12O40·xH2O) and various amounts of CNT conductive paste (5 wt%, LB217-54, Cnano technology Ltd) were dissolved in deionized water (500 mL) and vigorously stirred for 12 h. Then, the solution was treated with sonication for 1 h to obtain an homogeneous suspension. Subsequently, the above solution was treated by a commercial spray-drying procedure (SUNYI Spray Dryer SP-1500). The experimental parameters for the spray dryer were set as follows: pumping rate was 1000 mL h−1, the temperatures at the inlet and outlet of the spray dryer were 180 °C and 90 °C, respectively. The black powder was collected and further carbonized under Ar gas (100 sccm) at 800 °C for 5 h with a heating rate of 5 °C min−1 to obtain the W2C@CNT-S. For comparison, these W2C/CNT mixtures were synthesized using a similar process followed to obtain W2C@CNT-S, except that the mixtures were collected by oven drying instead of spray drying. In order to investigate the optimized weight ratio of W2C and CNT on HER performance, a series of samples were prepared with various molar ratios of C/W (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), with the corresponding products denoted as W2C@CNT-S6, W2C@CNT-S8, and W2C@CNT-S10, respectively.
1), with the corresponding products denoted as W2C@CNT-S6, W2C@CNT-S8, and W2C@CNT-S10, respectively.
2.2. Characterization
The phases of the W2C@CNT-S and W2C/CNT catalysts were identified by X-ray diffraction (XRD, Rigaku Ultima IV) with Cu Kα radiation (λ = 1.54178 Å). The chemical composition of the samples was identified using X-ray photoelectron spectroscopy (XPS, Kratos XSAM 800, Al Kα radiation). Raman spectrometry (Renishaw) was performed to obtain the Raman spectra of the samples with an excitation laser line of 532 nm in ambient conditions. The surface morphology and structures of samples were examined by using scanning electron microscopy (SEM, JSM-7000F, JEOL) and transmission electron microscopy (TEM, FEI Tecnai G2 F20) with an accelerating voltage of 200 kV. The chemical compositions, HAADF image, and corresponding elemental mapping of W and C were observed with energy X-ray spectroscopy (EDX).2.3. Electrochemical measurements
All the electrochemical experiments with the as-synthesized electrocatalysts for HER were conducted on an electrochemical workstation (CHI660D, CH Instruments Inc) with a standard three-electrode system at ambient temperature. A graphite rod and a saturated calomel electrode (SCE) were used as the counter electrode and reference electrode, respectively, in N2-saturated 0.5 M H2SO4. Also, experiments were performed in 1 M KOH, with a graphite rod and Hg/HgO electrode as the counter and reference electrodes, respectively. All the potentials were corrected versus a reversible hydrogen electrode (RHE) according to ERHE = ESCE + 0.255 V in 0.5 M H2SO4, ERHE = EHg/HgO + 0.93 V in 1 M KOH.12 Typically, 4 mg of the as-prepared catalyst was added in 1 mL of mixed solution (750 μL of water and 250 μL of ethanol), and then 60 μL Nafion (5 wt%) was added into the above solution. A homogeneous ink was obtained after 30 min of ultrasonication. Later, 5 μL of the catalyst ink was dropped onto the glassy carbon electrode (GCE, catalyst loading ∼0.28 mg cm−2) and dried in an oven at 60 °C. Linear sweep voltammetry (LSV) was performed in 0.5 M H2SO4 and 1 M KOH at a rate of 5 mV s−1 without iR-based correction. The double-layer capacitances (Cdl) were calculated through cyclic voltammograms (CV) at various sweep rates from 20 to 200 mV s−1. Electrochemical impedance spectroscopy (EIS) was carried out in the frequency range of 105 to 0.1 Hz.3. Results and discussion
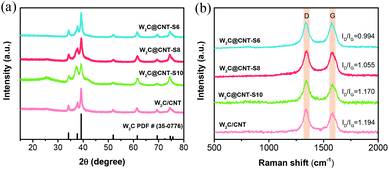
X-ray diffraction (XRD) was used to examine the chemical composition of the as-prepared W2C@CNT-S and W2C/CNT electrocatalysts, as shown in Fig. 2a. The characteristic diffraction peaks located at 34.5°, 37.9°, 39.5°, 52.1°, 61.8°, 69.7°, 72.8°, and 75.0° were assigned to the (100), (002), (101), (102), (110), (103), (200), and (112) crystal planes of a hexagonal W2C (PDF#35-0776), respectively, which was consistent with the previous reports that showed superior HER activities.23,24,28 No metallic W or WC was observed. The broad peaks at about 25.5° had relatively weak intensities. Fig. 2b shows the Raman spectra of W2C@CNT-S and W2C/CNT, with two distinct characteristic peaks located at 1356 cm−1 and 1580 cm−1, corresponding to the D band and G band of the carbon-based networks, respectively. The intensity ratio of D band and G band (ID/IG) can be considered as a characteristic parameter to reflect the degree of the carbon graphitization.29It is also known that the intensity ratio of the D band and G band (ID/IG) can be considered as a characteristic parameter to reflect the density of defects. The ID/IG ratio of pure spray-dried CNT (without carbonization) was 1.27 (Fig. S1†), which was higher than those of W2C@CNT-S6 (0.994), W2C@CNT-S8 (1.055), W2C@CNT-S10 (1.170), and W2C/CNT (1.194). That means, high-temperature carbonization was beneficial to decrease the density of defects; in addition, with the decreasing CNT content of W2C@CNT-S, the density of defects decreased.
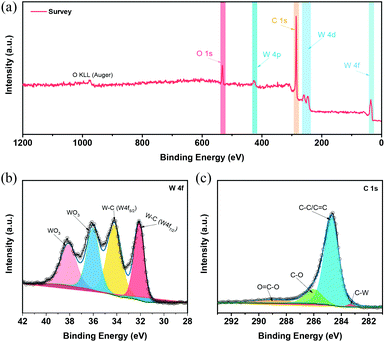
In this work, the optimized W2C@CNT-S8 with the best HER performance was utilized in all further characterizations and discussions. The chemical environment and valence state of W2C@CNT-S8 electrocatalyst was characterized through X-ray photoelectron spectroscopy (XPS). As observed, the XPS survey spectrum (Fig. 3a) identified the presence of the W, C, and O in the W2C@CNT-S8. The high-resolution of W 4f spectrumcan be seen in Fig. 3b, where the peaks located at binding energy of 32.1 and 34.2 eV are attributed to tungsten carbide (W 4f7/2 and W 4f5/2) in general.23,30 The peaks at binding energies of 36.0 and 38.1 eV result from the inevitable surface oxidation of tungsten carbide when exposed to air.19,20 According to the electrocatalytic results in Fig. S2,† we confirm that the WO3 had a negligible contribution to the HER and that the HER activity of W2C@CNT mainly originated from the W2C instead of WO3.30,31Fig. 3c displays the high-resolution C 1s XPS spectrum, with peaks at 283.2, 284.6, 286.0, and 289.0 eV, corresponding to the C–W, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (or C–C), C–O, and O
C (or C–C), C–O, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O bonds, respectively.31–33
C–O bonds, respectively.31–33
The morphologies and microstructures of the as-prepared products were observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As can be seen in Fig. S3,† the W2C/CNT hybrid shows a large size and the particles are not distributed homogeneously on the carbon nanotube because of the big clusters and severe aggregation during the oven-drying instead of spray-drying treatment, which indicates a small quantity of active sites for HER. The SEM images of pure CNT networks are also shown in Fig. S4,† showing a rough surface and uneven size of holes in the CNT three-dimensional (3D) networks after the spray-drying approach. These results indicate that the spray-drying approach and the carbon nanotubes as a carbon source can not only open up the possibility of more active sites and better electrical conductivity, but also provide for efficient electron transfer. As shown in Fig. 4a and b, one can clearly observe that the microsphere is composed of carbon nanotubes with a rough surface and that no large-sized particles or aggregation can be seen. Moreover, as can be seen from the high-resolution TEM images (Fig. 4c and d, and Fig. S5†), the ultrasmall W2C nanodots are homogeneously anchored on the surface of the CNTs. The formation of W2C nanodots through the diffusion of C atoms from the CNTs through the solid–solid interface into the W lattice is slowed down due to the chemical inertness of the multi-walled carbon nanotubes, which not only effectively limits the overgrowth of carbide nanodots by suppressing their agglomeration, but also avoids excessive carbon deposition formed on their surfaces.23,27 Furthermore, it has been reported that the nanodot materials show excellent performance and applicable prospects in electrochemistry and energy storage.27,32–35 The lattice fringes with an interplanar distance of 0.228 nm and 0.34 nm correspond to the (101) plane of hexagonal W2C and (002) plane of carbon nanotubes, respectively. Fig. 4e–g show the X-ray energy dispersive spectrometry (EDX) mapping images of one carbon nanotube decorated with W2C nanodots. From Fig. 4e–g, one can observe that the W and C are uniformly distributed into the composite, which verifies that there are homogeneous dispersion of W2C nanodots on the CNT networks.
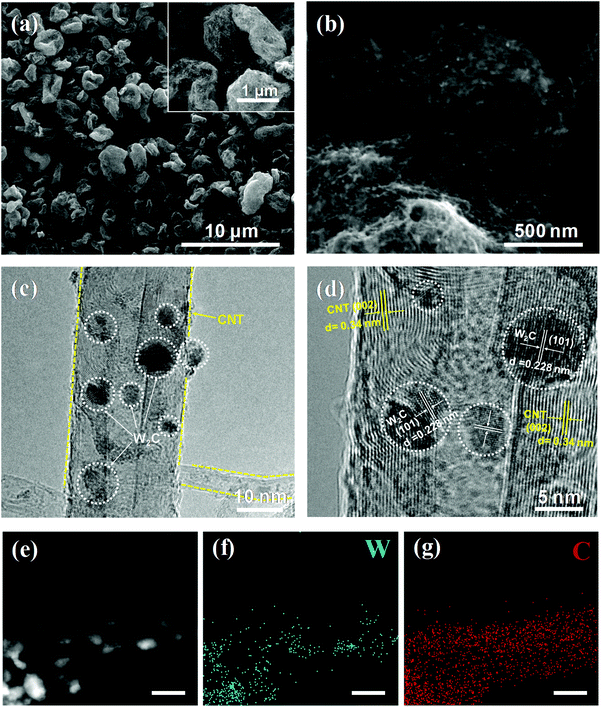 | ||
| Fig. 4 (a, b) SEM images (inset: scale bar of 1 μm), (c, d) HRTEM images, HAADF image (e) and corresponding W and C EDX element mappings (f, g) of W2C@CNT-S8 (with scale bar of 10 nm). | ||
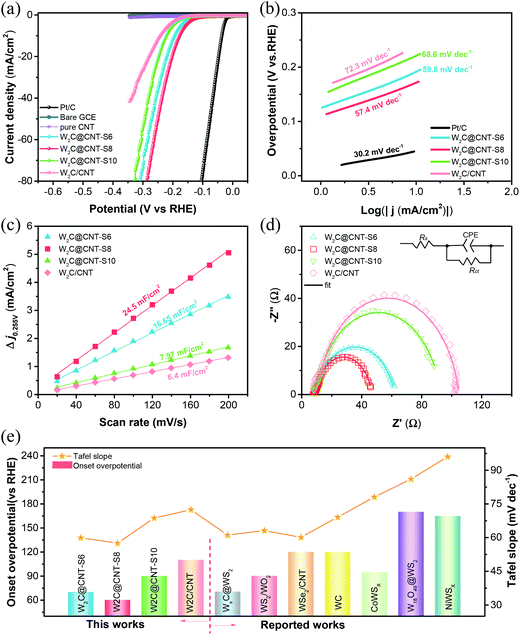
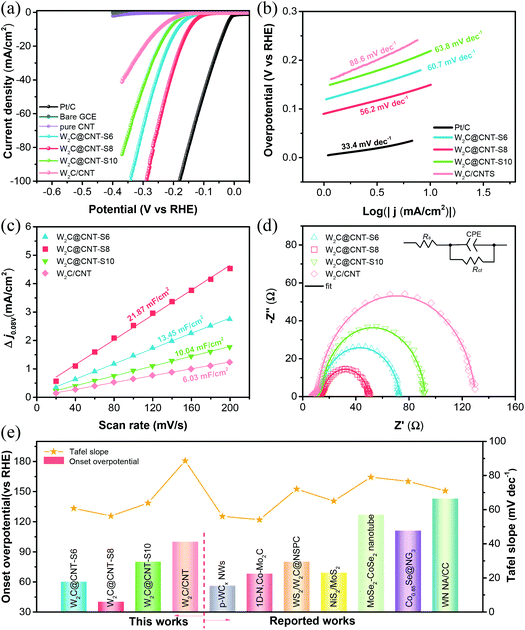
The electrocatalytic performance of the as-prepared W2C@CNT-S and W2C/CNT materials was first evaluated by linear sweep voltammetry (LSV) without iR-correction in 0.5 M H2SO4 solution. Simultaneously, the HER polarization curves of the bare GCE, commercial Pt/C (20 wt%), and the spray-dried pure CNT were also measured for comparison. As observed in Fig. 5a, the commercial Pt/C displayed the highest catalytic activity toward HER in acidic medium with nearly zero onset overpotential; whereas, the bare GCE showed very poor catalytic activity. The spray-dried pure CNT also had negligible HER activity in 0.5 M H2SO4. It was impressively found that the W2C@CNT-S electrocatalysts exhibited excellent performance, much better than the W2C/CNT catalyst. In order to investigate the optimized weight ratio of W2C and CNT on HER performance, a series of W2C@CNT-S samples prepared with various molar ratios of C/W (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 10
1, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were also tested through electrochemical measurements. According to the Fig. S6,† the optimized W2C@CNT-S8 exhibited a much smaller onset overpotential of 60 mV (vs. RHE) than the W2C/CNT (∼110 mV). Furthermore, it is well known that the overpotential at a current density of 10 mA cm−2 is another indispensable parameter for the HER.36,37 To obtain the current density of 10 mA cm−2, only about 176 mV, 192 mV, and 220 mV were needed for W2C@CNT-S8, W2C@CNT-S6, and W2C@CNT-S10, respectively, which were lower than that needed for W2C/CNT (240 mV). These results indicate that the HER activity mainly came from the W2C species. W2C@CNT-S6 did not display a better performance due to the lesser content of the CNT; while W2C@CNT-S10 containing a greater amount of CNT also did not show better performance than W2C@CNT-S8, due to the more CNT wrapped around the W2C nanodots, which limits exposure to the active sites. The aforementioned consequences imply that the as-synthesized W2C@CNT-S8 was comparable to those non-noble-metal-based HER electrocatalysts in acidic media, such as WxC@WS2,30 WS2/WO2,38 WC,39 NiWSx,40 CoWSx,40 W18O49@WS2,41 and WSe2/CNT42 (Fig. 5e, Table S1†).
1) were also tested through electrochemical measurements. According to the Fig. S6,† the optimized W2C@CNT-S8 exhibited a much smaller onset overpotential of 60 mV (vs. RHE) than the W2C/CNT (∼110 mV). Furthermore, it is well known that the overpotential at a current density of 10 mA cm−2 is another indispensable parameter for the HER.36,37 To obtain the current density of 10 mA cm−2, only about 176 mV, 192 mV, and 220 mV were needed for W2C@CNT-S8, W2C@CNT-S6, and W2C@CNT-S10, respectively, which were lower than that needed for W2C/CNT (240 mV). These results indicate that the HER activity mainly came from the W2C species. W2C@CNT-S6 did not display a better performance due to the lesser content of the CNT; while W2C@CNT-S10 containing a greater amount of CNT also did not show better performance than W2C@CNT-S8, due to the more CNT wrapped around the W2C nanodots, which limits exposure to the active sites. The aforementioned consequences imply that the as-synthesized W2C@CNT-S8 was comparable to those non-noble-metal-based HER electrocatalysts in acidic media, such as WxC@WS2,30 WS2/WO2,38 WC,39 NiWSx,40 CoWSx,40 W18O49@WS2,41 and WSe2/CNT42 (Fig. 5e, Table S1†).
The Tafel slope is an inherent parameter of electrocatalysts to investigate their kinetics mechanism and intrinsic activity with the HER process. As can be seen in Fig. 5b, the Tafel slopes of Pt/C, W2C@CNT-S6, W2C@CNT-S8, W2C@CNT-S10, and W2C/CNT were calculated from linear fitting of the polarization curves by the Tafel equation (η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a, where b is the Tafel slope and j is the current density). The Tafel slope of commercial Pt/C was 30.2 mV dec−1 in 0.5 M H2SO4, which is consistent with the reported literature.9,11,18 It is notable that W2C@CNT-S8 displayed a small Tafel slope of 57.4 mV dec−1, which was smaller than those of W2C@CNT-S6 (59.8 mV dec−1), W2C@CNT-S10 (68.6 mV dec−1), and W2C/CNT (72.3 mV dec−1). Generally, there are two main HER mechanisms in both acidic and alkaline media.26,36,49–51 In acidic media, the HER mechanism includes the following three steps.
j + a, where b is the Tafel slope and j is the current density). The Tafel slope of commercial Pt/C was 30.2 mV dec−1 in 0.5 M H2SO4, which is consistent with the reported literature.9,11,18 It is notable that W2C@CNT-S8 displayed a small Tafel slope of 57.4 mV dec−1, which was smaller than those of W2C@CNT-S6 (59.8 mV dec−1), W2C@CNT-S10 (68.6 mV dec−1), and W2C/CNT (72.3 mV dec−1). Generally, there are two main HER mechanisms in both acidic and alkaline media.26,36,49–51 In acidic media, the HER mechanism includes the following three steps.
Volmer reaction
| H3O+ + e− + catalyst → catalyst-Hads + H2O | (1) |
Heyrovsky reaction
| H3O+ + e− + catalyst-Hads → catalyst + H2 + H2O | (2) |
Tafel reaction
| 2 × Catalyst-Hads → 2 × catalysts + H2 | (3) |
Similarly, in alkaline media, the HER mechanism also includes the following three steps.
Volmer reaction
| H2O + e− + catalyst → catalyst-Hads + OH− | (4) |
Heyrovsky reaction
| H2O + e− + catalyst-Hads → catalyst + H2 + OH− | (5) |
Tafel reaction
| 2 × Catalyst-Hads → 2 × catalyst + H2 | (6) |
The W2C@CNT-S8 sample had the faster HER reaction kinetics owing to the smaller Tafel slope of 57.4 mV dec−1, demonstrating that the reaction of hydrogen evolution on the W2C@CNT-S8 followed the Volmer–Heyrovsky mechanism (Tafel slope range from 40 to 120 mV dec−1), which is the rate-determining step.26,36 These results indicate that the spray-drying method plays an important role in the preparation of W2C@CNT-S networks and in attaining an outstanding HER performance.
To further investigate the intrinsic differences for HER behavior among the catalysts, the electrochemical active surface area (ECSA), which is obtained from the double-layer capacitances (Cdl), was estimated. The cyclic voltammograms (CV) were measured at various scan rates in the range 0.205–0.305 V (vs. RHE) in 0.5 M H2SO4 solution (Fig. S7†). Fig. 5c shows that the double-layer capacitance (Cdl) of W2C@CNT-S8 was 24.5 mF cm−2 in 0.5 M H2SO4, which was the highest among those of W2C@CNT-S6 (16.65 mF cm−2), W2C@CNT-S10 (7.97 mF cm−2), and W2C/CNT (6.4 mF cm−2). The high Cdl value suggests the presence of additional active sites, which can enhance the transport of electrons for electrochemical processes. Furthermore, electrochemical impedance spectroscopy (EIS) was performed to investigate the favorable HER kinetics between W2C@CNT-S and W2C/CNT. Fig. 5d shows the corresponding Nyquist plots of these catalysts at −0.17 V vs. RHE in 0.5 M H2SO4. The charge-transfer resistance (Rct) was obtained from the fitting of the semicircles via an equivalent circuit model, which is shown in the inset. The Rct values are ranked as follows: W2C@CNT-S8 (37.83 Ω) < W2C@CNT-S6 (53.59 Ω) < W2C@CNT-S10 (83.38 Ω) < W2C/CNT (94.6 Ω), well in agreement with their Tafel slopes. The smaller Rct value of W2C@CNT-S8 suggests its faster electron kinetics for the HER.
The as-prepared W2C@CNT-S and W2C/CNT were also investigated as HER catalysts in alkaline media (1 M KOH). As demonstrated in Fig. 6, the HER performance of these samples was slightly enhanced in alkaline solution instead of acidic solution. As shown in Fig. 6a and Fig. S8,† the W2C@CNT-S8 exhibits superior performance with a smaller onset overpotential of 40 mV (vs. RHE), while the overpotential to reach 10 mA cm−2 was decreased to 148 mV, which was lower than that for W2C@CNT-S6 (186 mV), W2C@CNT-S10 (213 mV), and W2C/CNT (248 mV). In addition, Fig. 6b displays the Tafel slopes of 56.2 mV dec−1 for W2C@CNT-S8, 60.7 mV dec−1 for W2C@CNT-S6, 63.8 mV dec−1 for W2C@CNT-S10, and 88.6 mV dec−1 for W2C/CNT. The Tafel slope suggests the W2C@CNT-S8 catalyst proceeded through a Volmer–Heyrovsky mechanism.36,43 The double-layer capacitances of the as-prepared samples were examined by cyclic voltammograms at various scan rates ranging from 0.03 V to −0.13 V (vs. RHE) in 1 M KOH (Fig. S9†). According to Fig. 6c, the capacitances (Cdl) of W2C@CNT-S8 was 21.87 mF cm−2, which was higher than those of W2C@CNT-S6 (13.45 mF cm−2), W2C@CNT-S10 (10.04 mF cm−2), and W2C/CNT (6.03 mF cm−2), implying more active sites for the HER process. Fig. 6d shows the corresponding Nyquist plots of these catalysts by EIS measurement at −0.20 V vs. RHE. The Rct value of W2C@CNT-S8 (36.34 Ω) was much smaller than that of W2C@CNT-S6 (60.61 Ω), W2C@CNT-S10 (78.75 Ω), and W2C/CNT (119.4 Ω), indicating the faster electron-transfer rate of the W2C@CNT-S8 electrocatalyst for HER in alkaline solution, which was in excellent agreement with the aforementioned results of the HER activity. The W2C@CNT-S8 catalyst had an ultralow onset overpotential of 40 mV (vs. RHE) and a smaller Tafel slope of 56.2 mV dec−1 comparable to those reported for non-noble-metal-based electrocatalysts in alkaline media, such as p-WCx NWs,44 1D, Co-Mo2C,45 WS2/W2C@NSPC,46 NiS2/MoS2,47 MoSe2-CoSe2 nanotube,11 Co0.85Se@NG3,10 and WN NA/CC48 (Fig. 6e, Table S1†).
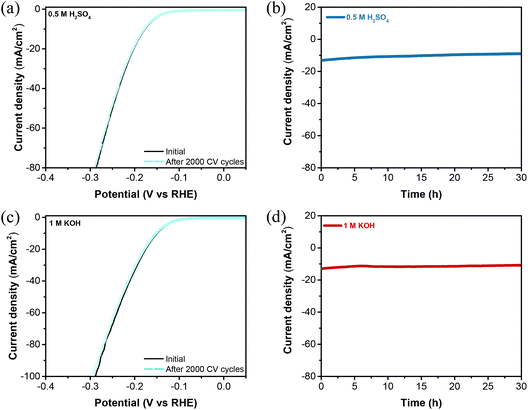
Durability is a crucial parameter for estimating the practical application of HER electrocatalysts. The long-term stability test of W2C@CNT-S8 was assessed by 2000 CV cycles at a scan rate of 100 mV s−1 in 0.5 M H2SO4 and 1 M KOH. As shown in Fig. 7a and c, the polarization curves of the W2C@CNT-S8 catalyst showed negligible decay of the cathodic current after 2000 cycles of CV under both acidic and alkaline electrolytes, indicating their excellent stability. Meanwhile, chronoamperometric (CA) measurements were performed for W2C@CNT-S8 at a constant overpotential of −180 mV and −200 mV in 0.5 M H2SO4 and 1 M KOH electrolyte, respectively. As shown in Fig. 7b and d, the I–t plots show that the current density exhibited negligible degradation after continuous testing for over 30 h in both 0.5 M H2SO4 and 1 M KOH, respectively. We further characterized the W2C@CNT-S8 after the HER stability test in both acidic and alkaline solutions. As shown in Fig. S10–S13,† the SEM and TEM images indicate that the porous network structure of W2C@CNT-S8 was maintained nearly unchanged even after the long-term stability tests in both alkaline and acidic solutions, which means the W2C@CNT-S8 electrocatalyst is a promising catalyst for practical application.
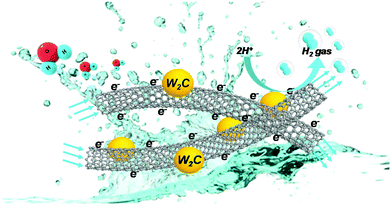
Moreover, the electrocatalytic process of W2C@CNT-S is schematically illustrated in Fig. 8. In this process, the electrons can transfer through the CNTs to W2C nanodots anchored on the 3D and conductive CNT skeleton. Hydrogen ions at the exposed active sites of W2C nanodots electrocatalyst are reduced by the transferred electrons, followed by the release of hydrogen gas. As mentioned above, the excellent and efficient electrocatalytic activity and durability of W2C@CNT-S toward HER could be attributed to the following aspects: (i) the spray-drying method can create porous CNT networks, as a carbon framework, which can lead to the uniform dispersion of W2C nanodots, which provide more active sites to enhance the HER catalytic activity; (ii) the CNT skeleton ensures outstanding electrical conductivity and prevents the W2C nanodots from being aggregated; (iii) due to the chemical inertness of CNT, the diffusion rate of C atom from carbon nanotubes into the W lattice is slowed down, not only effectively limiting the overgrowth of W2C particles to ensure the formation of W2C nanodots, but also preventing extensive carbon deposition formed on the surface of tungsten carbide;23,27,52 (iv) the robust contact between W2C and CNT facilitates the efficient electron-transfer rate and enables excellent durability for the hydrogen evolution reaction.
4. Conclusions
In this work, we first developed a scalable and cost-efficient method to synthesize ultrasmall W2C nanodots decorated on CNT networks via a spray-drying process (designated as W2C@CNT-S). Compared to the W2C/CNT (obtained without spray drying), the optimized W2C@CNT-S8 exhibited better HER performance with a lower onset overpotential of 60 or 40 mV (vs. RHE), a smaller Tafel slope of 57.4 or 56.2 mV dec−1, and a current density@10 mA cm−2 of 176 or 148 mV (vs. RHE) in acidic and alkaline media, respectively. Besides, it exhibited outstanding stability after long-term operation for over 30 h in both 0.5 M H2SO4 and 1 M KOH. The superior HER performance in both acidic and alkaline solution could be attributed to the CNT network structure and ultrafine W2C nanodots homogenously decorated on conductive CNT networks, which expose sufficient active sites and promote high-efficiency electron transfer for electrochemical reactions. This work presents a novel, low-cost, and scalable strategy, which can be extended to design other nonprecious-metal-based carbides with efficient activity for the hydrogen evolution reaction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was supported by the National Natural Science Foundation of China (Grant No. 21773024, 51372033), and National High Technology Research and Development Program of China (Grant No. 2015AA034202).Notes and references
- J. A. Turner, Science, 2004, 305, 972 CrossRef CAS PubMed.
- M. S. Faber and S. Jin, Energy Environ. Sci., 2014, 7, 3519 RSC.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148 RSC.
- M. R. Gao, Y. F. Xu, J. Jiang and S. H. Yu, Chem. Soc. Rev., 2013, 42, 2986 RSC.
- J. Wang, F. Xu, H. Jin, Y. Chen and Y. Wang, Adv. Mater., 2017, 29, 1605838 CrossRef PubMed.
- Z. Wang, Q. Li, H. Xu, C. Dahl-Petersen, Q. Yang, D. Cheng, D. Cao, F. Besenbacher, J. V. Lauritsen, S. Helveg and M. Dong, Nano Energy, 2018, 49, 634 CrossRef CAS.
- J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. D. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807 CrossRef CAS PubMed.
- S. Lu, X. Shang, L. Zhang, B. Dong, W. Gao, F. Dai, B. Liu, Y. Chai and C. Liu, Appl. Surf. Sci., 2018, 445, 445 CrossRef CAS.
- B. Yu, F. Qi, B. Zheng, W. Hou, W. Zhang, Y. Li and Y. Chen, J. Mater. Chem. A, 2018, 6, 1655 RSC.
- H. Wang, X. Wang, D. Yang, B. Zheng and Y. Chen, J. Power Sources, 2018, 400, 232 CrossRef CAS.
- X. Wang, B. Zheng, B. Yu, B. Wang, W. Hou, W. Zhang and Y. Chen, J. Mater. Chem. A, 2018, 6, 7842 RSC.
- B. Wang, Z. Wang, X. Wang, B. Zheng, W. Zhang and Y. Chen, J. Mater. Chem. A, 2018, 6, 12701 RSC.
- J. Ren, L. Chen, C. Weng, G. Yuan and Z. Yuan, ACS Appl. Mater. Interfaces, 2018, 10, 33276 CrossRef CAS PubMed.
- T. Wu, M. Pi, X. Wang, W. Guo, D. Zhang and S. Chen, Appl. Surf. Sci., 2018, 427, 800 CrossRef CAS.
- H. Yan, Y. Xie, Y. Jiao, A. Wu, C. Tian, X. Zhang, L. Wang and H. Fu, Adv. Mater., 2018, 30, 1704156 CrossRef PubMed.
- Y. Zhong, X. Xia, F. Shi, J. Zhan, J. Tu and H. J. Fan, Adv. Sci., 2016, 3, 1500286 CrossRef PubMed.
- W. F. Chen, C. H. Wang, K. Sasaki, N. Marinkovic, W. Xu, J. T. Muckerman, Y. Zhu and R. R. Adzic, Energy Environ. Sci., 2013, 6, 943 RSC.
- Y. Hu, D. Guan, B. Yu, W. Hou, B. Zheng, W. Zhang and Y. Chen, Electrochim. Acta, 2018, 263, 192 CrossRef CAS.
- N. Han, K. R. Yang, Z. Lu, Y. Li, W. Xu, T. Gao, Z. Cai, Y. Zhang, V. S. Batista, W. Liu and X. Sun, Nat. Commun., 2018, 9, 924 CrossRef PubMed.
- Y. Xu, X. Xiao, Z. Ye, S. Zhao, R. Shen, C. He, J. Zhang, Y. Li and X. Chen, J. Am. Chem. Soc., 2017, 139, 5285 CrossRef CAS PubMed.
- Z. Zhao, F. Qin, S. Kasiraju, L. Xie, M. K. Alam, S. Chen, D. Wang, Z. Ren, Z. Wang, L. C. Grabow and J. Bao, ACS Catal., 2017, 7, 7312 CrossRef CAS.
- S. Emin, C. Altinkaya, A. Semerci, H. Okuyucu, A. Yildiz and P. Stefanov, Appl. Catal., B, 2018, 236, 147 CrossRef CAS.
- Q. Gong, Y. Wang, Q. Hu, J. Zhou, R. Feng, P. N. Duchesne, P. Zhang, F. Chen, N. Han, Y. Li, C. Jin, Y. Li and S. Lee, Nat. Commun., 2016, 7, 13216 CrossRef CAS PubMed.
- L. Zhang, Y. Ma, Z. Lang, Y. Wang, S. U. Khan, G. Yan, H. Tan, H. Zang and Y. Li, J. Mater. Chem. A, 2018, 6, 15395 RSC.
- A. Kurlov and A. Gusev, Inorg. Mater., 2006, 42, 121 CrossRef CAS.
- H. Wei, Q. Xi, X. Chen, D. Guo, F. Ding, Z. Yang, S. Wang, J. Li and S. Huang, Adv. Sci., 2018, 1700733 CrossRef PubMed.
- B. Yu, D. Yang, Y. Hu, J. He, Y. Chen and W. He, Small Methods, 2018, 1800287 Search PubMed.
- G. Yan, C. Wu, H. Tan, X. Feng, L. Yan, H. Zang and Y. Li, J. Mater. Chem. A, 2017, 5, 765 RSC.
- F. Malara, S. Carallo, E. Rotunno, L. Lazzarini, E. Piperopoulos, C. Milone and A. Naldoni, ACS Catal., 2017, 7, 4786 CrossRef CAS.
- F. Wang, P. He, Y. Li, T. A. Shifa and Y. Deng, Adv. Funct. Mater., 2017, 27, 1605802 CrossRef.
- I. Kim, S. Park and D. Kim, Nanoscale, 2018, 10, 21123 RSC.
- C. Tan, Z. Luo, A. Chaturvedi, Y. Cai, Y. Du, Y. Gong, Y. Huang, Z. Lai, X. Zhang, L. Zheng, X. Qi, M. H. Goh, J. Wang, S. Han, X. Wu, L. Gu, C. Kloc and H. Zhang, Adv. Mater., 2018, 30, 1705509 CrossRef PubMed.
- Z. Lai, A. Chaturvedi, Y. Wang, T. H. Tran, X. Liu, C. Tan, Z. Luo, B. Chen, Y. Huang, G. Nam, Z. Zhang, Y. Chen, Z. Hu, B. Li, S. Xi, Q. Zhang, Y. Zong, L. Gu, C. Kloc, Y. Du and H. Zhang, J. Am. Chem. Soc., 2018, 140, 8563 CrossRef CAS PubMed.
- M. Wang, C. Tang, C. Ye, J. Duan, C. Li, Y. Chen, S. Bao and M. Xu, J. Mater. Chem. A, 2018, 6, 14734 RSC.
- K. Chang, H. Pang, X. Hai, G. Zhao, H. Zhang, L. Shi, F. Ichihara and J. Ye, Appl. Catal., B, 2018, 232, 446 CrossRef CAS.
- Y. Zhang, Y. Wang, C. Han, S. Jia, S. Zhou and J. Zang, Nanoscale, 2017, 9, 19176 RSC.
- B. Yu, Y. Hu, F. Qi, X. Wang, B. Zheng, K. Liu, W. Zhang, Y. Li and Y. Chen, Electrochim. Acta, 2017, 242, 25 CrossRef CAS.
- J. Wang, W. Wang, Z. Wang, J. G. Chen and C. Liu, ACS Catal., 2016, 6, 6585 CrossRef CAS.
- H. Fei, Y. Yang, X. Fan, G. Wang, G. Ruan and J. M. Tour, J. Mater. Chem. A, 2015, 3, 5798 RSC.
- P. D. Tran, S. Y. Chiam, P. P. Boix, Y. Ren, S. S. Pramana, J. Fize, V. Artero and J. Barber, Energy Environ. Sci., 2013, 6, 2452 RSC.
- B. Seo, H. Y. Jeong, S. Y. Hong, A. Zak and S. H. Joo, Chem. Commun., 2015, 51, 8334 RSC.
- X. Wang, Y. Chen, B. Zheng, F. Qi, J. He, P. Li and W. Zhang, Electrochim. Acta, 2016, 222, 1293 CrossRef CAS.
- J. Xiong, J. Li, J. Shi, X. Zhang, N. Suen, Z. Liu, Y. Huang, G. Xu, W. Cai, X. Lei, L. Feng, Z. Yang, L. Huang and H. Cheng, ACS Energy Lett., 2018, 3, 341 CrossRef CAS.
- B. Ren, D. Li, Q. Jin, H. Cui and C. Wang, J. Mater. Chem. A, 2017, 5, 13196 RSC.
- J. Wan, J. Wu, X. Gao, T. Li, Z. Hu, H. Yu and L. Huang, Adv. Funct. Mater., 2017, 27, 1703933 CrossRef.
- Y. Li, X. Wu, H. Zhang and J. Zhang, ACS Appl. Energy Mater., 2018, 1, 3377 CrossRef CAS.
- P. Kuang, T. Tong, K. Fan and J. Yu, ACS Catal., 2017, 7, 6179 CrossRef CAS.
- J. Shi, Z. Pu, Q. Liu, A. M. Asiri, J. Hu and X. Sun, Electrochim. Acta, 2015, 154, 345 CrossRef CAS.
- P. Kuang, B. Zhu, Y. Li, H. Liu, J. Yu and K. Fan, Nanoscale Horiz., 2018, 3, 317 RSC.
- H. Gu, W. Fan and T. Liu, Nanoscale Horiz., 2017, 2, 277 RSC.
- S. Zhang, X. Xiao, T. Lv, X. Lv, B. Liu, W. Wei and J. Liu, Appl. Surf. Sci., 2018, 446, 10 CrossRef CAS.
- Z. Chen, L. Duan, T. Sheng, X. Lin, Y. Chen, Y. Chu, S. Sun and W. Lin, ACS Appl. Mater. Interfaces, 2017, 9, 20594 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nr10281c |
| This journal is © The Royal Society of Chemistry 2019 |