Open Access Article
Open Access ArticleSuper-resolution microscopy on single particles at fluid interfaces reveals their wetting properties and interfacial deformations†
A.
Aloi
 *abc,
N.
Vilanova
ac,
L.
Isa
*abc,
N.
Vilanova
ac,
L.
Isa
 d,
A. M.
de Jong
d,
A. M.
de Jong
 ae and
I. K.
Voets
ae and
I. K.
Voets
 *abcf
*abcf
aInstitute for Complex Molecular Systems, Eindhoven University of Technology, Post Office Box 513, 5600 MB Eindhoven, The Netherlands. E-mail: I.Voets@tue.nl; Antonio.Aloi@tue.nl
bLaboratory of Self-Organizing Soft Matter, Department of Chemistry and Chemical Engineering, Eindhoven University of Technology, Post Office Box 513, 5600 MB Eindhoven, The Netherlands
cLaboratory of Macromolecular and Organic Chemistry, Department of Chemistry and Chemical Engineering, Eindhoven University of Technology, Post Office Box 513, 5600 MB Eindhoven, The Netherlands
dLaboratory for Interfaces, Soft Matter and Assembly, Department of Materials, ETH Zurich, Vladimir-Prelog Weg 5, 8093 Zürich, Switzerland
eLaboratory of Molecular Biosensing, Department of Applied Physics, Eindhoven University of Technology, Post Office Box 513, 5600 MB Eindhoven, The Netherlands
fLaboratory of Physical Chemistry, Department of Chemistry and Chemical Engineering, Eindhoven University of Technology, Post Office Box 513, 5600 MB Eindhoven, The Netherlands
First published on 13th March 2019
Abstract
Solid particles adsorbed at fluid interfaces are crucial for the mechanical stability of Pickering emulsions. The key parameter which determines the kinetic and thermodynamic properties of these colloids is the particle contact angle, θ. Several methods have recently been developed to measure the contact angle of individual particles adsorbed at liquid–liquid interfaces, as morphological and chemical heterogeneities at the particle surface can significantly affect θ. However, none of these techniques enables the simultaneous visualization of the nanoparticles and the reconstruction of the fluid interface to which they are adsorbed, in situ. To tackle this challenge, we utilize a newly developed super-resolution microscopy method, called iPAINT, which exploits non-covalent and continuous labelling of interfaces with photo-activatable fluorescent probes. Herewith, we resolve with nanometer accuracy both the position of individual nanoparticles at a water–octanol interface and the location of the interface itself. First, we determine single particle contact angles for both hydrophobic and hydrophilic spherical colloids. These experiments reveal a non-negligible dependence of θ on particle size, from which we infer an effective line tension, τ. Next, we image elliptical particles at a water–decane interface, showing that the corresponding interfacial deformations can be clearly captured by iPAINT microscopy.
Introduction
Materials comprising homogeneous mixtures of immiscible fluids are of paramount importance for a broad range of natural and technological processes, but they all suffer from an unavoidable, thermodynamically driven propensity to phase-separate. One way to halt the macroscopic phase separation of two immiscible liquids makes use of the adsorption of micro- and nanoparticles at the fluid interface. It is the kinetic trapping of colloidal particles of various shape,1–3 roughness,4–6 softness,7–11 and surface chemistry12 at fluid interfaces, which grants long-term stability to particle-stabilized emulsions.13,14 This enhances their mechanical properties and offers adequate protection against coalescence.15–17Although particle-laden interfaces have been the subject of systematic investigations for more than a century,13,14 only recently new methods have emerged as alternative to ensemble measurements to interrogate wetting properties at the single-particle level. The primary challenge for a precise estimation of the contact angle is the accurate localization of the adsorbed colloids and of the interface. Recent developments of methods with high spatial resolution and image contrast, such as interferometric microscopy,18,19 freeze-fracture shadow-casting cryo-scanning electron microscopy (FreSCa),5,20–22 digital holography,23,24 Bessel beam microscopy,25 and intra-pair magnetophoresis,26,27 allowed directly observing single particles and measuring their vertical position with respect to the fluid interface. In addition to conventional contact-angle measurements, these new tools revealed that particle heterogeneities (e.g. chemical or shape anisotropies, surface functionalization, roughness) have a large impact on the stabilization of fluid interfaces.5,28 In particular, one could finally extrapolate interfacial energy landscapes, and pinpoint where particle detachments would be energetically more favoured,25,29 and lead to mechanical instabilities.30 Moreover, these could identify where particle dynamics would differ due to changes in local viscosity,31–33 and when capillary forces would dominate assembly.34,35
These important insights illustrate the potential of the newly developed tools despite limitations in e.g. particle size and sample preparation. Some of the approaches require fixation of the (particles at the) interface to perform ex situ SEM or AFM imaging with nanometric spatial resolution. Fluid phases are for instance gelled and peeled-off in the gel trapping technique (GTT) to examine the particles’ position,36–38 which may perturb their equilibrium position at the interface due to the applied mechanical forces.39 Moreover, GTT presents limited accuracy for objects smaller than 100 nm, since the gel's structural features are of the order of 10 nm. In an alternative approach, the position of the three-phase contact line (TPCL) is marked using specific chemical reactions for a more gentle identification.40 The contact angle of single particles as small as 10 nm can be determined by FreSCa microscopy.20,21,41,42 Its complex sample preparation comprises three steps of shock-freezing the fluid interface, exposing the particles upon fracturing the interface, and subsequently coating the colloids with a thin metal layer at a given metal-casting angle, α. FreSCa is well-suited for hydrophobic particles, but not for extremely hydrophilic particles with contact angles lower than the accessible range of metal-casting angles (i.e., θ < αcr, with αcr = 30–45°).20 Moreover, a three-dimensional reconstruction of the interface surrounding the particles is not possible, and, as only part of the particles is visible, θ is extracted through geometrical assumptions on the particle shape.
Here, we present an innovative method which allows truly in situ imaging without any external fixation required of single, immobilized nanoparticles adsorbed at fluid interfaces and the simultaneous reconstruction of the interface to which they are adsorbed. To accomplish this, we exploit a single-molecule localization microscopy (SMLM)43 technique known as interface Point Accumulation for Imaging in Nanoscale Topography (iPAINT), which was recently developed in our group.44 This strategy exploits the interfacial adsorption of a photo-activatable dye end-attached to the water-soluble polymer polyethylene glycol (PEG-552) for the imaging of soft materials with <20 nm resolution (Fig. S2†).45–48 Advantageously, iPAINT enables simultaneous visualization of both particles and interface via a simple, non-covalent staining approach; i.e., physical adsorption of suitable dyes. Disadvantageously, this impacts the local force balance and hence affects e.g. the position of the particles at the interface, such that their contact angles are altered by ±10° (Fig. S12 and S13†).
We first demonstrate the visualization by iPAINT of the position of hydrophobic and hydrophilic spherical silica nanoparticles at the water–octanol interface. Next, we explore the wettability of the particles, determining their mean contact angles, θ, and related distributions. We find a non-negligible dependence of individual θ on particle's size that can be ascribed to line tension effects. Finally, we image local deformations of the fluid interface induced by the adsorption of anisotropic particles. These findings demonstrate the potential of our innovative approach to advance the characterization and understanding of the role of particles on interface stabilization, which may aid the rational design of (nano)particles for Pickering emulsions.
Results and discussion
In situ imaging of colloidal particles at the interface between immiscible liquids
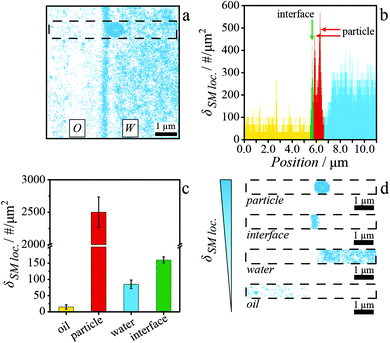
An accurate measurement of the wetting of individual particles adsorbed at fluid interfaces requires a precise localization of the particles’ and interface's position. To critically evaluate whether iPAINT can be utilized for this purpose, we set out to image two extensively characterized systems:49 plain hydrophilic and stearyl alcohol-grafted hydrophobic silica particles (Fig. S4 and S5†) at water–octanol interfaces. First, iPAINT probes (PEG-552) are solubilized in water and the colloids are dispersed in either the water or the oil phase, in accordance with their wettability. Next, water and oil droplets with the particles and iPAINT probes are placed side-by-side on a coverglass and subsequently compressed by a coverslip positioned on top to orient the water–oil interfaces normal to the coverslip, as schematically illustrated in Fig. 1a and b. This allows us to profit from the high accuracy in single molecule localization in the lateral plane (∼17 nm). In the assembled sample chamber, a dynamic equilibrium is now established, wherein PEG-552 is predominantly solubilized in the aqueous phase and physically adsorbed at all the water-accessible interfaces (Fig. 1b): the water–oil interface, as well as the surface of the particles and the coverslip exposed to the water phase. To resolve the position of individual dyes with high precision, we tune the fraction of emitting dyes to several tens in a frame of ∼1900 μm2 by regulating the power of the photo-activation and the readout lasers (refer to Materials and Methods section, Fig. 1c and Fig. S1†). Conveniently, Brownian motion precludes PEG-552 localization in solution, while the individual labels are readily identified when adsorbed at interfaces where their motion is restricted (i.e., particle surface, fluid interface and microscope coverslip).44 Consequentially, all water-accessible interfaces are effectively stained. In a final step of image analysis, all single molecule localizations are summed up to yield a super-resolved reconstruction of the particles at the fluid interface.An exemplary iPAINT image of a hydrophilic particle adsorbed at an octanol–water interface is presented in Fig. 2a. A pronounced difference in single-molecule localization density is apparent, which we exploit for unambiguous identification of the relevant interfaces. The stark contrast originates from the low respectively high solubility of PEG-552 in oil and water, which leads to an uneven partitioning between the two phases and the particle–water–octanol interfaces (the single-molecule localizations observed in the water and oil phase in Fig. 2a belong to the dye molecules adsorbed on the microscope coverslip). Additionally, particles and fluid interfaces show a different density of single-molecule localization being projected on a 2D plane. First, an area is selected, which spans both the octanol and water phases and is as wide as one and as long as fourteen particle diameters (700 nm × 10 μm, demarcated by a dashed line in Fig. 2a). Next, the density of single-molecule localizations per μm2, δSM loc., is determined along the 10 μm cut (Fig. 2b) to obtain δSM loc. for the oil and water phases, the water–octanol interface, and both types of particles. The procedure is repeated for tens of measurements, which gives the averaged δSM loc. and associated standard deviations, as reported in Fig. 2c. These mean values are hereafter used as threshold parameters to discriminate between the four localization categories. These are subsequently split into four separate datasets in descending order of δSM loc. to analyse and display exclusively those localizations belonging to the particles (Fig. 2d, top row), the interface (Fig. 2d, second row), the water phase (Fig. 2d, third row), and the oil phase (Fig. 2d, bottom row). This procedure enables the localization with high accuracy (<20 nm) of the position of both hydrophilic and hydrophobic particles relative to the interface (Fig. S6 and S7†).
Determining the contact angle of single hydrophilic and hydrophobic particles at an oil–water interface
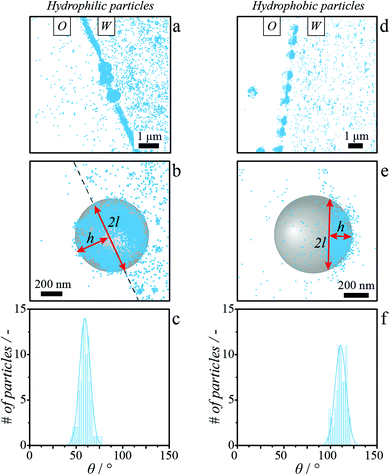
The simultaneous visualization by iPAINT of spherical particles and the water–octanol interface to which they adsorb, enables the determination of the contact angle, θ, of single particles (Fig. 3).To this end, the height of the spherical cap protruding through the interface, h, and its lateral dimension, 2l, are obtained from iPAINT reconstructions in an analogous yet slightly different manner for hydrophilic and hydrophobic particles. Hydrophilic colloids are rather uniformly covered by PEG-552 (Fig. 3a and b), because these particles approached the interface from the aqueous phase. We therefore overlay the localization datasets belonging to the interface and to the particle to determine h and 2l (Fig. 3b and Fig. S6†). Hydrophobic particles, which were dispersed in octanol and thus approached the interface from the oil phase, are only partially labelled with PEG-552 where the particle surface has been exposed to water (Fig. 3d and e). Both h and 2l are thus directly determined from the localization datasets corresponding to the stearyl alcohol-coated beads (Fig. 3e and Fig. S7†). In line with the differences in surface functionalization and thus wettability of the two types of spherical particles with equal mean size, we obtain much smaller θ values for the hydrophilic particles (θ = 59.5° ± 6.9°, Fig. 3c) than for the hydrophobic particles (θ = 111.5° ± 7.0°, Fig. 3f). The single-particle values are also in agreement with the contact angles reported for μm-sized silica beads at water–octanol interfaces as obtained from ensemble measurements (θhydrophilic = 68° ± 6°,49θhydrophobic = 148° ± 5°,49 and Fig. S14†). We observe a modest broadening of the contact angle distribution, σθ, due to particle size dispersity and possibly chemical heterogeneities of 5.5° and 2.3° for hydrophilic and hydrophobic particles, respectively (Fig. S9 and S10†). The interested reader is referred to the ESI† for an in-depth discussion of the computation of the single particle contact angles (eqn (S1) and eqn (S3)†), aging of the TPCL (Fig. S8†),50 and the impact of chemical heterogeneities, size dispersity51 (Rhydrophobic = 329 ± 21 nm, Rhydrophilic = 328 ± 17 nm) and iPAINT experimental resolution (Fig. S9†) on σθ.
Recent single-particle experiments and simulations revealed that θ values for sub-μm particles at fluid interfaces scale linearly with particle size.38 This is because θ values must be corrected for non-negligible contributions due to the line tension, τ, which corresponds to the force exerted on the particle around the TPCL.38
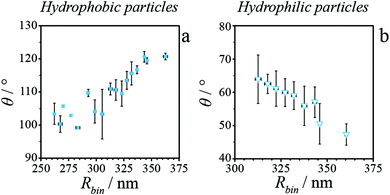
To evaluate the size-dependence of θ for hydrophilic and hydrophobic silica particles studied herein, we replot in Fig. 4 the single particle contact angle values as a function of their sizes. For clarity, θ values are averaged for particles differing less than 5 nm in mean radius (i.e., ΔRbin ≤ 5 nm, Fig. S11†). For hydrophobic particles (Fig. 4a), we recover the expected trend of increasing θ with increasing particle radius. Hydrophilic particles exhibit the opposite trend of increasing θ with decreasing particle radius (Fig. 4b). We may now compute τ from the iPAINT data using the Young–Dupré equation
 | (1) |
Visualizing local interfacial deformations induced by adsorbed elliptical particles
The in situ visualization of both adsorbed particles and liquid–liquid interfaces opens up the exciting prospect of imaging by iPAINT local deformations of the fluid interface induced by particle adsorption. To assess this possibility, we performed iPAINT experiments on ellipsoidal polystyrene particles (Fig. S15†) adsorbed at water–decane interfaces. It is well-known that such anisotropic particles distort the liquid–liquid interface to which they are adsorbed to locally satisfy Young's equation by pulling up the liquid along the major axis, and pushing it down at their tips (Fig. 5a).55–58 Gratifyingly, local interfacial deformations around ellipsoids straddling a water-decane interface at the equilibrium position are indeed resolved by iPAINT (Fig. 5d and Fig. S16†). From iPAINT images, we first determine the contact angle of single ellipsoids, θ (Fig. S18†), and then quantify the maximal interfacial deformation, Δumax, at the tips of the particle (Fig. 5b). Δumax is normalized by half the minor axis of each ellipsoid, a, to account for the dispersity in particle size. In agreement with theoretical predictions,56,59 we find that Δumax increases non-monotonically with θ (Fig. 5f) and goes to zero at 90° (dashed line in Fig. 5f). We find that Δumax reaches a maximum value at θ = 43.4°, in accordance with modelling studies by Lehle et al. and Dasgupta et al. predicting a maximum at θ = 46° (ref. 55) and θ = 48.5°,59 respectively. | ||
| Fig. 5 Deformation of the oil–water interface due to the presence of anisotropic particles. (a) Schematic of deformations induced by an ellipsoidal particle at the equilibrium position straddling a fluid interface. Δumax is the maximum displacement of the three-phase contact line. (b, c) Schematic showing the interfacial deformation (blue line) compared to an unperturbed interface (red line) for a (b) parallel and (c) tilted ellipsoid. (d, e) Exemplary iPAINT images of deformed fluid interfaces in presence of (d) parallel and (e) tilted ellipsoidal particles thereby adsorbed. The orange line along the ellipsoids perimeter and the red line used to define the fluid interface are determined by α-shaping.62 Maximum difference in the contact line height, Δumax, as a function of the (f) contact angle, θ, and of the (g) polar angle, φ, for parallel and oblique orientations of the ellipsoids, respectively. The dashed line in (f) shows the theoretical values from Dasgupta et al.59 The dashed-dotted line in (g) is meant to guide the eye. The error bars represent the standard deviation. | ||
Surprisingly, we also observe ellipsoids in an oblique orientation at fluid interfaces (Fig. 5e and Fig. S16, S17†). These particles are captured in a non-equilibrium state, which we define as an ‘arrested configuration’. We speculate that this particular orientation occurs in our experiments as particles settle and are immobilized on the coverslip while pivoting within the interface towards their equilibrium position (Fig. S19†).
Simulations on ellipsoids with ferromagnetic dipoles in oblique orientations revealed a depression of the interface on one side and an elevation on the opposite side (Fig. 5c).60,61 For a comparison of our findings with those results, we first define the tilting (polar) angle, φ, of the ellipsoid as the angle between the interface and the major axis of the colloid (Fig. S16†). We then determine the maximum deformation, Δumax, which the interface can adopt as the distance along the surface normal between the outermost single-molecule localizations on the interface, close to the tips of the ellipsoid in the oil and in the water phase, respectively (Fig. 5c). In good agreement with computations,63,64 this reveals a non-monotonic dependence of Δumax/a as a function of φ, which reaches a maximum at φ = 38.7° and decreases to zero at φ = 90° (Fig. 5g). These findings clearly demonstrate that iPAINT is a valuable complementary tool to visualize in situ colloidal particles at liquid–liquid interfaces as well as the interfacial deformations thereby induced, offering access to quantitative information on single particle contact angles and local interfacial perturbations.
Experimental
Materials and methods
Hydrophilic silica beads of ∼300 nm in radius were synthesized using a two-step Stöber-based method.65 Silica particles with intermediate hydrophobicity were obtained by partially functionalizing them with stearyl alcohol through a modified van Helden method (Fig. S4†).66 Polystyrene ellipsoidal particles were obtained by stretching monodisperse sulfate latex spheres of 1 μm in diameter (Invitrogen) while embedded into a poly(vinyl alcohol) (PVA) matrix above the glass transition temperature and below the melting temperature of the material (Fig. S15†).67 Here, prolate ellipsoids with aspect ratio of 5 were used.22
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v concentrated H2SO4
1 v/v concentrated H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2(aq. 30%)) to remove impurities, and reduce the background fluorescence. Solvent evaporation was not encountered during image acquisition due to the short imaging time-lapses (∼20 min for a 256 × 256 pixels field of view).
H2O2(aq. 30%)) to remove impurities, and reduce the background fluorescence. Solvent evaporation was not encountered during image acquisition due to the short imaging time-lapses (∼20 min for a 256 × 256 pixels field of view).
Being normal to the focal plane, and squeezed between two surfaces, the oil–water interface appeared μm-wide, depending on the angle of the quasi-total internal reflection fluorescence (TIRF) illumination adopted in the experiment. This is because the interfacial plane is not perfectly normal to the coverglass along the entire thickness of the spacers used. In other words, the interface is somewhat curved on μm length scale. However, the angle in the quasi-TIRF was set to illuminate the first μm's of the interface in contact with the coverslip. In this small penetration depth of the laser, we can assume that the interface is normal to the coverslip.
Conclusions
In this work, we present the design of an innovative and complementary method based on super-resolution microscopy to image colloidal particles and fluid interfaces simultaneously and in situ. Through the continuous and non-covalent physisorption of polymer-conjugated photoactivatable dyes onto interfaces, we investigate the wetting properties of single hydrophobic and hydrophilic colloidal particles at a liquid–liquid interface, with a spatial resolution <20 nm. From iPAINT images, contact angle distributions and mean contact angles are determined, clearly showing a size-dependence of θ. Using the modified Young–Dupré equation, we account for this size dependence by calculating effective line tension forces exerted on the particles in the nanoNewton range. The possibility to follow the contour of the interface with high spatial resolution encouraged us to investigate further the interfacial deformations caused by the adsorption of anisotropic particles. iPAINT imaging of ellipsoidal colloids at decane–water interfaces shows the interface dipping at the tips and rising along the sides of the particles, confirming earlier theoretical computations on bigger ellipsoids. The minimally invasive and simultaneous imaging of interfaces and particles combined with its high spatial resolution makes iPAINT microscopy the ideal candidate to interrogate in situ phenomena connected to particles at interfaces. In particular, we hope to address further questions concerning the specific role of particle's size, shape and surface properties, which are essential to provide a full understanding of the assembly of particles at fluid interfaces.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Prof. Jan Vermant for useful discussion, M. M. R. M Hendrix for AFM measurements and C. C. M. Sproncken for interfacial tension experiments. This work was supported by the Dutch Science Foundation (NWO VIDI Grant 723.014.006), the European Union (ERC 2014 StG contract 635928), and the Dutch Ministry of Education, Culture and Science (Gravity program 024.001.035).Notes and references
- R. G. Alargova, D. S. Warhadpande, V. N. Paunov and O. D. Velev, Langmuir, 2004, 20, 10371 CrossRef CAS PubMed.
- B. Madivala, S. Vandebril, J. Fransaer and J. Vermant, Soft Matter, 2009, 5, 1717–1727 RSC.
- T. M. Ruhland, A. H. Gröschel, N. Ballard, T. S. Skelhon, A. Walther, A. H. E. Müller and S. A. F. Bon, Langmuir, 2013, 29, 1388–1394 CrossRef CAS PubMed.
- A. San-Miguel and S. H. Behrens, Langmuir, 2012, 28, 12038–12043 CrossRef CAS PubMed.
- M. Zanini, C. Marschelke, E. S. Anachkov, E. Marini, A. Synytska and L. Isa, Nat. Commun., 2017, 8, 15701 CrossRef CAS PubMed.
- M. Zanini, I. Lesov, E. Marini, C.-P. Hsu, C. Marschelke, A. Synytska, S. E. Anachkov and L. Isa, Langmuir, 2018, 34, 4861–4873 CrossRef CAS PubMed.
- A. Rauh, M. Rey, L. Barbera, M. Zanini, M. Karg and L. Isa, Soft Matter, 2017, 13, 158–169 RSC.
- R. W. Style, L. Isa and E. R. Dufresne, Soft Matter, 2015, 11, 7412–7419 RSC.
- K. Geisel, L. Isa and W. Richtering, Langmuir, 2012, 28, 15770–15776 CrossRef CAS PubMed.
- O. S. Deshmukh, D. van den Ende, M. A. Cohen Stuart, F. Mugele and M. H. G. Duits, Adv. Colloid Interface Sci., 2015, 222, 215–227 CrossRef CAS PubMed.
- V. Schmitt and V. Ravaine, Curr. Opin. Colloid Interface Sci., 2013, 18, 532–541 CrossRef CAS.
- M. Xiao, A. Xu, T. Zhang and L. Hong, Front. Chem., 2018, 6, 225 CrossRef PubMed.
- S. U. Pickering, J. Chem. Soc. Trans., 1907, 91, 2001–2021 RSC.
- W. Ramsden, Proc. R. Soc. London, 1903, 72, 156–164 CAS.
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, A. R. Bausch, M. Marquez and D. A. Weitz, Science, 2002, 298, 1006–1009 CrossRef CAS PubMed.
- E. Bormashenko, Curr. Opin. Colloid Interface Sci., 2011, 16, 266–271 CrossRef CAS.
- E. M. Herzig, K. A. White, A. B. Schofield, W. C. K. Poon and P. S. Clegg, Nat. Mater., 2007, 6, 966–971 CrossRef CAS PubMed.
- J. C. Loudet and B. Pouligny, Europhys. Lett., 2009, 85, 28003 CrossRef.
- J. C. Loudet and B. Pouligny, Phys. Rev. Lett., 2006, 97, 018304 CrossRef CAS PubMed.
- L. Isa, F. Lucas, R. Wepf and E. Reimhult, Nat. Commun., 2011, 2, 438 CrossRef PubMed.
- B. P. Binks, L. Isa and A. T. Tyowua, Langmuir, 2013, 29, 4923–4927 CrossRef CAS PubMed.
- S. Coertjens, P. Moldenaers, J. Vermant and L. Isa, Langmuir, 2014, 30, 4289–4300 CrossRef CAS PubMed.
- J. Fung, R. W. Perry, T. G. Dimiduk and V. N. Manoharan, J. Quant. Spectrosc. Radiat. Transfer, 2012, 113, 212–219 CrossRef.
- A. Wang, W. B. Rogers and V. N. Manoharan, Phys. Rev. Lett., 2017, 119, 108004 CrossRef PubMed.
- C. Snoeyink, S. Barman and G. F. Christopher, Langmuir, 2015, 31, 891–897 CrossRef CAS PubMed.
- S. Cappelli, A. M. de Jong, J. Baudry and M. W. J. Prins, Soft Matter, 2016, 12, 5551–5562 RSC.
- S. Cappelli, A. M. de Jong, J. Baudry and M. W. J. Prins, Langmuir, 2017, 33, 696–705 CrossRef CAS PubMed.
- C. J. Mable, N. J. Warren, K. L. Thompson, O. O. Mykhaylyk and S. P. Armes, Chem. Sci., 2015, 6, 6179–6188 RSC.
- P. I. Pieranski, Phys. Rev. Lett., 1980, 45, 569–572 CrossRef CAS.
- F. Bresme and M. Ottel, J. Phys.: Condens. Matter, 2007, 17, 413101 CrossRef PubMed.
- T. M. Squires and T. G. Mason, Annu. Rev. Fluid Mech., 2010, 42, 413–438 CrossRef.
- Y. Peng, W. Chen, T. M. Fischer, D. A. Weitz and P. Tong, J. Fluid Mech., 2009, 618, 243–261 CrossRef CAS.
- T. M. Fischer, P. Dhar and P. Heinig, J. Fluid Mech., 2006, 558, 451–475 CrossRef.
- T. Cubaud and T. G. Mason, Phys. Fluids, 2008, 20, 053302 CrossRef.
- S. L. Anna, Annu. Rev. Fluid Mech., 2016, 48, 285–309 CrossRef.
- V. N. Paunov, Langmuir, 2003, 19, 7970–7976 CrossRef CAS.
- L. N. Arnaudov, O. J. Cayre, M. A. Cohen Stuart, S. D. Stoyanov and V. N. Paunov, Phys. Chem. Chem. Phys., 2010, 12, 328–331 RSC.
- S. P. McBride and B. M. Law, Phys. Rev. Lett., 2012, 109, 196101 CrossRef PubMed.
- B. M. Law, S. P. McBride, J. Y. Wang, H. S. Wie, G. Paneru, S. Betelu, B. Ushijima, Y. Takata, B. Flanders, F. Bresme, H. Matsubara, T. Takiue and M. Aratono, Prog. Surf. Sci., 2017, 92, 1–39 CrossRef CAS.
- M. Sabapathy, V. Kollabattula, M. G. Basavaraj and E. Mani, Nanoscale, 2015, 7, 13868–13876 RSC.
- J. van Rijssel, M. van der Linden, J. D. Meeldijk and R. J. A. van Dijk-Moes, Phys. Rev. Lett., 2013, 111, 108302 CrossRef PubMed.
- N. Vogel, J. Ally, K. Bley, M. Kappl, K. Landfester and C. K. Weiss, Nanoscale, 2014, 6, 6879–6885 RSC.
- A. Aloi and I. K. Voets, Curr. Opin. Colloid Interface Sci., 2018, 34, 59–73 CrossRef CAS.
- A. Aloi, N. Vilanova, L. Albertazzi and I. K. Voets, Nanoscale, 2016, 8, 8712–8716 RSC.
- A. Aloi, C. Guibert, L. L. C. Olijve and I. K. Voets, Polymer, 2016, 107, 450–455 CrossRef CAS.
- B. Adelizzi, A. Aloi, N. J. Van Zee, A. R. A. Palmans, E. W. Meijer and I. K. Voets, ACS Nano, 2018, 12, 4431–4439 CrossRef CAS PubMed.
- N. J. Van Zee, B. Adelizzi, M. F. J. Mabesoone, X. Meng, A. Aloi, R. H. Zha, M. Lutz, I. A. W. Filot, A. R. A. Palmans and E. W. Meijer, Nature, 2018, 558, 100–103 CrossRef CAS.
- B. Adelizzi, A. Aloi, A. J. Markvoort, H. M. M. Ten Eikelder, I. K. Voets, A. R. A. Palmans and E. W. Meijer, J. Am. Chem. Soc., 2018, 140, 7168–7175 CrossRef CAS PubMed.
- A. Maestro, L. J. Bonales, H. Ritacco, R. G. Rubio and F. Ortega, Phys. Chem. Chem. Phys., 2010, 12, 14115–14120 RSC.
- D. M. Kaz, R. McGorty, M. Mani, M. P. Brenner and V. N. Manoharan, Nat. Mater., 2012, 11, 138–142 CrossRef CAS PubMed.
- M. Zanini and L. Isa, J. Phys.: Condens. Matter, 2016, 28, 313002 CrossRef PubMed.
- A. Maestro, E. Guzmán, F. Ortega and R. G. Rubio, Curr. Opin. Colloid Interface Sci., 2014, 19, 355–367 CrossRef CAS.
- M. Shao, J. Wang and X. Zhou, Sci. Rep., 2015, 5, 9491 CrossRef CAS PubMed.
- J. H. Weijs, A. Marchand, B. Andreotti, D. Lohse and J. H. Snoeijer, Phys. Fluids, 2011, 23, 022001 CrossRef.
- H. Lehle, E. Noruzifar and M. Ottel, Eur. Phys. J. E: Soft Matter Biol. Phys., 2008, 26, 151–160 CrossRef CAS PubMed.
- M. Oettel and S. Dietrich, Langmuir, 2008, 24, 1425–1441 CrossRef CAS PubMed.
- J. Vermant, Nature, 2011, 476, 286–287 CrossRef CAS PubMed.
- J. C. Loudet, A. M. Alsayed, J. Zhang and A. G. Yodh, Phys. Rev. Lett., 2005, 94, 018301 CrossRef CAS PubMed.
- S. Dasgupta, M. Katava, M. Faraj, T. Auth and G. Gompper, Langmuir, 2014, 30, 11873–11882 CrossRef CAS PubMed.
- L. Botto, E. P. Lewandowski Jr., M. Cavallaro and K. J. Stebe, Soft Matter, 2012, 8, 9957–9971 RSC.
- G. B. Davies, T. Krüger, P. V. Coveney, J. Harting and F. Bresme, Adv. Mater., 2014, 26, 6715–6719 CrossRef CAS PubMed.
- S. Lou, X. Jiang and P. J. Scott, Proc. R. Soc. A, 2013, 469, 20130150 CrossRef.
- G. B. Davies and L. Botto, Soft Matter, 2015, 11, 7969–7976 RSC.
- G. B. Davies, T. Krüger, P. V. Coveney, J. Harting and F. Bresme, Soft Matter, 2014, 10, 6742–6748 RSC.
- N. Vilanova, I. de Feijter and I. K. Voets, J. Visualized Exp., 2016, 110, e53934 Search PubMed.
- A. K. van Helden, J. W. Jansen and A. Vrij, J. Colloid Interface Sci., 1981, 81, 354–368 CrossRef CAS.
- K. M. Keville, E. I. Franses and J. M. Caruthers, J. Colloid Interface Sci., 1991, 144, 103–126 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of particles, iPAINT data analysis, macroscopic characterization of the system, Fig.: S1–S19. See DOI: 10.1039/c8nr08633h |
| This journal is © The Royal Society of Chemistry 2019 |