Redox active mixed-valence hexamanganese double-cubane complexes supported by tetravanadates†
Abstract
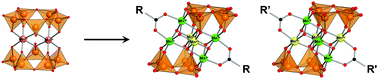
A mixed-valence hexamanganese complex, [MnIII4MnIV2O6(V4O13)2(MeCO2)2]6− [1(MeCO2)], has been synthesized by the reaction of a dimanganese precursor, [Mn2(V5O15)2]6− (Mn2V10), with (n-Bu4N)MnO4 and Mn(MeCO2)2 in acetonitrile. Compound 1(MeCO2) possesses a face-sharing double-cubane {MnIII4MnIV2O6} core that is sandwiched by two [V4O13]6− units with two bridging acetate groups. Several benzoate derivatives, [Mn6O6(V4O13)2(RCO2)]6− [1(RCO2), R = Ph, p-ClPh, p-MePh, p-NO2Ph], are also synthesized to study the substituent effects and the redox potentials were well-correlated with Hammett constants. In complex 1(MeCO2), four manganese atoms at the edges are assigned as being in a trivalent state and two manganese atoms at the center are assigned as being in a tetravalent state from X-ray crystallography. One electron oxidation product, [MnIII3MnIV3(V4O13)2(RCO2)2]5−, is also isolated for the benzoate derivatives of [2(RCO2), R = p-MePh, o-MePh]. The arrangements of Mn(III) and Mn(IV) sites depending on the oxidation state of the hexamanganese core and type of functional group on the acetate group were investigated by a crystallographic study along with a magnetic susceptibility study.
- This article is part of the themed collection: Vanadium Science: Chemistry, Catalysis, Materials, Biological and Medicinal Studies


 Please wait while we load your content...
Please wait while we load your content...