Catalytic methane oxidation by a supramolecular conjugate based on a μ-nitrido-bridged iron porphyrinoid dimer†
Yasuyuki
Yamada
 *abc,
Kentaro
Morita
b,
Nozomi
Mihara
*abc,
Kentaro
Morita
b,
Nozomi
Mihara
 b,
Kazunobu
Igawa
b,
Kazunobu
Igawa
 d,
Katsuhiko
Tomooka
d,
Katsuhiko
Tomooka
 d and
Kentaro
Tanaka
d and
Kentaro
Tanaka
 *a
*a
aDepartment of Chemistry, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8602, Japan. E-mail: yy@chem.nagoya-u.ac.jp; kentaro@chem.nagoya-u.ac.jp
bResearch Center for Materials Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8602, Japan
cJST, PRESTO, 4-1-8 Honcho, Kawaguchi, Saitama, 332-0012, Japan
dInstitute for Materials Chemistry and Engineering, and IRCCS, Kyushu University, Kasuga-Koen, Kasuga, Fukuoka, 816-8580, Japan
First published on 14th June 2019
Abstract
Catalytic methane oxidation was conducted using a μ-nitrido-bridged dinuclear iron complex of a four-fold rotaxane heterodimer of a porphyrin and a phthalocyanine. Extension of the π-stacked structure of the four-fold rotaxane-based μ-nitrido-bridged iron porphyrinoid dimer by supramolecular complexation with an additional tetraanionic porphyrin apparently increased the methane conversion ability.
Introduction
Methane is the main constituent of natural gas. Following the “shale gas revolution,” significant efforts have been made to develop methods for the direct and efficient conversion of methane into more valuable raw materials such as MeOH, formaldehyde, and formic acid.1 On the other hand, since methane has a particularly high C–H bond dissociation energy (105 kcal mol−1) among the family of chemically inert light alkanes,2 efficient and direct low-temperature C–H activation of methane has been recognized as a long-standing challenge in the field of catalytic chemistry.3–8In natural systems, oxygenases such as soluble methane monooxygenase (sMMO), butane monooxygenase, and cytochrome P450 achieve the low-temperature efficient conversion of light alkanes by utilizing high-valent iron-oxo species.9–16 These examples have encouraged researchers to produce a variety of biomimetic iron-oxo species and develop a rich research field of iron-oxo-based molecular systems.17–21 However, only a few bio-inspired iron-oxo species are capable of oxidizing methane catalytically under mild reaction conditions.22,23 Some iron-oxo species lack the oxidizing ability, while others are susceptible to a variety of deactivation reactions, including oxidation of organic ligands and solvents and dimerization to form unreactive species.
Despite these bottlenecks, Sorokin et al. found that a μ-nitrido-bridged iron phthalocyanine dimer (FePc(tBu)4)2N is capable of oxidizing light alkanes in acidic aqueous solution, in the presence of H2O2 at low temperature (<100 °C).24–28 They reported that an ultra-high-valent iron-oxo species, which was generated by the reaction of (FePc(tBu)4)2N with H2O2, was the actual reactive species in this reaction. (FePc(tBu)4)2N has been recognized as one of the most potent molecular-based methane-oxidation catalysts reported so far.
We recently reported the supramolecular activation of the ethane oxidation activity of a μ-nitrido-bridged dinuclear iron porphyrinoid dimer-based catalyst. For this purpose, we designed a μ-nitrido-bridged dinuclear iron complex constructed in a four-fold rotaxane heterodimer of a porphyrin and a phthalocyanine prepared by using 4,8-diazacyclononyne (DACN) as a terminal stopper (15+·5Cl− in Fig. 1). Since 15+·5Cl− possesses four peripheral ammonium cations, the addition of a tetraanionic metalloporphyrin (M-TPPS4−, 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin metal complex, M = Cu(II) or Ni(II)) resulted in the extension of the stacked structure to form 15+-M-TPPS4−·Cl− through π–π stacking and quadruple electrostatic interactions, as shown in Fig. 1. The ethane oxidation activity of 15+-M-TPPS4−·Cl− in an acidic aqueous solution, in the presence of H2O2 at 60 °C, was almost twice that determined before complexation with M-TPPS4−.29 Thus, 15+-M-TPPS4−·Cl− showed higher catalytic ethane oxidation activity than (FePc(tBu)4)2N. Herein, we first applied our four-fold rotaxane-based μ-nitrido-bridged iron porphyrinoid dimer to the most difficult light alkane oxidation, that is, the methane oxidation reaction. Moreover, we showed that the catalytic methane oxidation reaction was activated by the supramolecular extension of the π-stacked structure of the four-fold rotaxane-based catalyst.
Results and discussion
A μ-nitrido-bridged dinuclear iron complex of a four-fold rotaxane heterodimer 15+·5Cl− and its stacked assemblies with M-TPPS4− (M = Cu2+: 15+-Cu(II)-TPPS4−·Cl− and M = Ni2+: 15+-Ni(II)-TPPS4−·Cl−) were synthesized according to our previous report.29 Catalytic methane oxidation reactions using these catalysts were performed in an acidic aqueous solution in the presence of H2O2. H2O is a suitable solvent for this reaction because the catalysts can decompose most organic solvents, including DMF and CH3CN.2,24 Since the catalysts were insoluble in H2O, they were pre-adsorbed on silica gel to prepare solid-supported catalysts. It has already been confirmed by UV-Vis spectroscopy that the stacked structures of 15+ with M-TPPS4− do not change during adsorption on SiO2.29We first investigated the products of the methane oxidation reaction using 15+·5Cl−/SiO2 by 1H NMR spectroscopy. The heterogeneous oxidation of methane was performed using 15+·5Cl−/SiO2 (141 μM as 15+·5Cl−) in D2O (1.5 mL) containing H2O2 (160 mM) and trifluoroacetic acid (TFA, 51 mM) under a methane atmosphere (1.0 MPa) in a 10 mL reaction vessel at 60 °C for 8 h. TFA-acidic conditions are useful for alkane oxidation using the metal ion in the presence of H2O2 as well as H2SO4-acidic conditions.8,30 In this case, acidic conditions are necessary for acceleration of the generation of high-valent iron-oxo species via protonation of iron-hydroperoxo species.24,25 The 1H NMR spectrum of the solution is shown in Fig. 2. After the reaction, a signal corresponding to formic acid was observed at 8.26 ppm. The signals at 5.07 and 3.89 were assignable as formaldehyde methyl hemiacetal, whereas signals due to formaldehyde monohydrate were not observed.31,32 This indicated that formaldehyde generated in situ reacted with MeOH under the acidic conditions. A very small signal attributed to free MeOH was observed at 3.38 ppm under these conditions. These oxidation reactions did not proceed in the absence of H2O2 and were very slow in the absence of TFA. Moreover, these oxidized compounds were formed in apparently smaller amounts or were not observed in the absence of methane, as shown in Fig. S1 (ESI†). These results clearly indicated that 15+·5Cl− acted as a methane oxidation catalyst under the present reaction conditions to produce formic acid, formaldehyde, and methanol. Formic acid observed in the absence of methane might have derived from the organic compounds adsorbed on SiO2, such as organic solvents. The actual reactive species should be the high-valent iron-oxo complex generated in situ,24,27 which was previously observed by performing electrospray-ionization Fourier transform ion cyclotron resonance mass spectroscopy (ESI-FT-ICR MS).29
 | ||
| Fig. 2 1H NMR spectra of the reaction mixture for methane (1.0 MPa) oxidation by 15+·5Cl−/SiO2 (141 μM as 15+·5Cl−) in the presence of H2O2 (160 mM) and TFA (51 mM) in D2O at 60 °C for 8 h. | ||
Based on these results, we next investigated the time dependence of the concentrations of each product of the catalytic methane oxidation using 1+·5Cl−/SiO2. After the reaction in H2O (1.5 mL) in the presence of 15+·5Cl−/SiO2 (71 μM), H2O2 (160 mM), and trifluoroacetic acid (TFA, 51 mM) under a methane atmosphere (1.0 MPa) in a 10 mL reaction vessel at 60 °C, the reaction mixture was analyzed by GC–MS. Formic acid and MeOH were successfully quantified by direct injection of the resulting solution into the GC–MS system. MeOH was found to be dissociated from formaldehyde under the GC–MS conditions. However, since direct quantification of formaldehyde was difficult, we derivatized formaldehyde into an O-alkyloxime via a reaction with O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine (PFBHA) for GC–MS quantification, according to a report by Yu et al.33,34
The results of GC–MS analysis are summarized in Fig. 3 and Table S1 (entries 1–10) in the ESI.† A gradual increase in the amount of formic acid was observed during the course of the reaction. The concentrations of MeOH and formaldehyde were much lower than that of formic acid even at the initial stage of the reaction (after 1 h). This indicated that the rates of oxidation of MeOH into formaldehyde and formaldehyde into formic acid were much higher than those of methane into MeOH and formic acid into CO2. We calculated the total turnover number (TTNeff) and the methane conversion number (MCNeff) as indicators of the reaction progress. TTNeff is defined by eqn (i) and (ii), where C represents the concentration of each species. This is based on the reaction shown in Fig. 3(a), where methane was oxidized stepwise into formic acid through MeOH and formaldehyde. On the other hand, MCNeff is defined by eqn (iii) and (iv). MCNeff indicates the number of methane molecules converted into MeOH using a single catalyst molecule, whereas TTNeff reflects the number of H2O2 molecules consumed by the single catalyst molecule for a series of oxidations of methane and its oxidized products. In order to calculate the effective TTN or MCN (TTNeff or MCNeff) for methane oxidation, TTN (MCN) under a N2 atmosphere was subtracted from TTN (MCN) under a CH4 atmosphere.
| TTNeff = TTN(CH4) − TTN(N2) | (i) |
| TTN(CH4) or TTN(N2) = (CMethanol + 2 × CFormaldehyde + 3 × CFormic acid)/CCat | (ii) |
| MCNeff = MCN(CH4) − MCN(N2) | (iii) |
| MCN(CH4) or MCN(N2) = (CMethanol + CFormaldehyde + CFormic acid)/CCat | (iv) |
TTNeff increased almost linearly up to 8 h, as shown in Fig. 3(b), indicating that the catalyst was not degraded and worked stably during this reaction time. However, after 8 h of reaction, TTNeff was saturated. Considering that catalyst degradation hardly occurred for 24 h ethane oxidation under similar reaction conditions, the decrease in the methane oxidation rate was not attributed to catalyst degradation26 but to the overoxidation of formic acid into CO2. Although a direct observation of the amount of CO2 generated during the reaction was difficult, we confirmed that formic acid was gradually oxidized, as shown in Fig. S2 (ESI†). This indicated that the oxidizing ability of 15+·5Cl− is so strong that even formic acid can be oxidized. Moreover, catalase activity might also contribute to the decrease in the catalytic activity by consuming H2O2.35 Furthermore, TTNeff after 8 h of methane oxidation was almost one-fourth of the value for ethane oxidation under the same conditions (Table S1, run 11, ESI†). This reflected the higher C–H bond dissociation energy of methane than that of ethane. The possible reaction mechanism for methane oxidation by 15+ is shown in Fig. 4.29 This mechanism is composed of two reaction cycles and the reactive intermediates are the high-valent iron-oxo species 1-c and 1-d. In our previous paper, we confirmed that homolytic cleavage of the O–O bonding of the hydroperoxo species (1-b to 1-d) hardly occurred under these reaction conditions.29
 | ||
| Fig. 4 Possible reaction mechanism for methane oxidation by the μ-nitrido-bridged dinuclear iron complex 15+. | ||
The methane oxidation ability of supramolecular stacked assemblies (15+-Cu(II)-TPPS4−·Cl− and 15+-Ni(II)-TPPS4−·Cl−) was examined in the same manner with 15+·5Cl− at 60 °C using a silica-supported catalyst (Fig. 536 and Table S1 (runs 12, 13), ESI†). As in the case of 15+, the supramolecular conjugate was stable at least up to 24 h.29 The MCNeff values for 15+-Cu(II)-TPPS4− and 15+-Ni(II)-TPPS4− were 18 and 19 after 8 h, which were almost 1.4 times higher than those before complexation. The same trend was observed for TTNeff. The oxo-species of the conjugate should be formed in the iron porphyrin center because Cu(II)-TPPS4− fully covers the iron center of the phthalocyanine unit in 15+.37,3815+-Ni(II)-TPPS4− showed a similar reactivity to that of the 15+-Cu(II)-TPPS4− conjugate, although Cu(II)-TPPS4− had a spin of S = 1/2 while Ni(II)-TPPS4− had no spin (S = 0) in its metal center. This suggests that the effect of the central metal ion of TPPS4− is not significant despite the close and direct contact of M-TPPS4− with the Fe–N–Fe center of 15+.
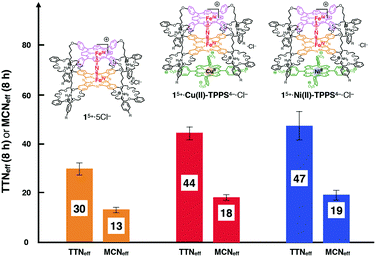 | ||
| Fig. 5 A comparison of MCNeff after 8 h oxidation of methane by a μ-nitrido-bridged iron porphyrinoid dimer-based catalyst on silica supports in the presence of H2O2 at 60 °C. Error bars indicate standard deviation of three measurements.36 | ||
In our previous study on ethane oxidation, we observed a more apparent (almost two-fold) enhancement of the catalytic activity after the formation of the stacked assembly with M-TPPS4−.29 We attributed this enhancement to electron donation through the π–π stacking of 15+ with M-TPPS4− because the redox potentials of both the 1e− redox waves of Fe(III)/Fe(IV) and π/π+ showed negative shifts (difference in the redox potentials before and after complexation (ΔE) was up to 0.03 V and 0.02 V, respectively, in CH2Cl2 solutions including 0.1 M nBu4N+PF6−). Sorokin reported that a μ-nitrido-bridged phthalocyanine dimer with an electron-donating substituent showed a much higher methane oxidizing ability than that with an electron-withdrawing substituent.25 Moreover, the push effect of the monomeric iron porphyrin is well known, i.e., electron-donating substituents can facilitate the generation of a reactive oxo species by enhancing the release of the OH− group from the hydroperoxo-iron species.39–42 In Fig. 4, this release of OH− corresponds to the conversion from 1-b to 1-c or from 1-f to 1-d. However, the degree of enhancement in the case of methane oxidation was significantly lesser than that of ethane oxidation. This difference implies that electron donation via the stacked assembly formation mainly contributes to an increase in the rate of generation of the reactive oxo species (1-c or 1-d in Fig. 4), but does not necessarily increase the reactivity of the oxo species.
Conclusions
Herein, we investigated the methane oxidation reaction by utilizing μ-nitrido-bridged iron porphyrinoid dimer-based catalysts constructed in a porphyrin–phthalocyanine heterodimer connected via a four-fold rotaxane structure. Methane was catalytically oxidized into MeOH, formaldehyde, and formic acid in an acidic aqueous solution, in the presence of H2O2. Since the four-fold rotaxane heterodimer scaffold has four peripheral ammonium cations, the addition of a tetraanionic porphyrin resulted in close stacking on the phthalocyanine side of the heterodimer. This complexation enhanced the methane oxidation ability of the μ-nitrido-bridged iron porphyrinoid dimer by increasing the generation rate of the reactive oxo species via electron donation through π–π stacking. The findings of this study might contribute to the development of more potent oxidizing catalysts based on iron-oxo species.Experimental
General
All reagents and solvents were purchased at the highest commercial quality available and used without further purification, unless otherwise stated. The four-fold rotaxane 15+·5Cl− and its stacked assemblies with M-TPPS4− (15+-Cu(II)-TPPS4−·Cl− and 15+-Ni(II)-TPPS4−·Cl−) were prepared according to our previous report.29Preparation of silica-supported catalysts. Silica-supported catalysts were prepared according to the reported procedure.26 In a typical method, 15+·5Cl− (2.81 μmol) was dissolved in 15 mL of CH2Cl2. After the addition of silica gel (473 mg) to the solution, CH2Cl2 was evaporated, and the resulting 15+·5Cl−/SiO2 was dried under vacuum at 60 °C for 8 h.
1H NMR analysis of the reaction mixture after CH4 oxidation by 15+·5Cl−
Methane oxidation was performed in a stainless-steel autoclave with a glass tube. A mixture of the catalyst/SiO2 (211 nmol of the Fe complex), TFA (6.0 μL, 78 μmol), and 30% H2O2 aq. (25 μL, 245 μmol) in D2O (1.5 mL) was heated at 60 °C under 1.0 MPa of methane for 8 h. After the reaction, the mixture was filtrated through a disposable membrane filter, and the filtrate was subjected to NMR analysis using [2,2,3,3,-D4] sodium 3,3-(trimethylsilyl)propanate (TSP) in D2O as an external standard. NMR spectra were recorded using a JEOL ECA600 (600 MHz for 1H) spectrometer at a constant temperature of 298 K.The methane oxidation reaction
Methane oxidation was performed in a stainless-steel autoclave with a glass tube. A mixture of the catalyst/SiO2 (106 nmol of the Fe complex), TFA (6.0 μL, 78 μmol), and 30% H2O2 aq. (25 μL, 245 μmol) in H2O (1.5 mL) was heated at 60 °C under 1.0 MPa of methane for 1–24 h. After the reaction, the mixture was filtrated through a disposable membrane filter, and the resulting filtrate was analyzed by GC–MS (system: Agilent 7890A equipped with JEOL JMS-T100GCV, detection: EI, column: Agilent DB-WAX UI, external standard: isovaleric acid (5 mM), and temperature conditions: initial: 70 °C to 220 °C (10 °C min−1) – hold (5 min)). The yields of methanol and formic acid were determined based on the results of GC–MS. The yield of formaldehyde was determined using the method reported by Yu et al.31,32 Typically, 25 μL of the filtrate obtained from the reaction mixture was diluted with 50 mL of H2O, followed by the addition of an aqueous solution (469 μM) of PFBOA·HCl (3.0 mL). The resulting mixture was stirred for 2 h. Then, sulfuric acid (1 + 1) (0.8 mL), NaCl (20 g), and hexane (5.0 mL) were added, and the mixture was stirred vigorously for 5 min. The separated organic layer was dried over anhydrous Na2SO4. A mixture of the resulting solution (1.0 mL) and 1.0 mM 1-chlorodecane/hexane solution (10.1 μL) was analyzed by GC–MS.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (A) (Number 19H00902) to KT, a JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (Number 19H02787), JST PRESTO (Number JK114b), the Shorai Science Foundation for Science and Technology for YY. YY and KI acknowledge support from the MEXT project of “Integrated Research Consortium on Chemical Sciences”. We are also grateful to Prof. Y. Ohki, Prof. O. Shoji, Dr S. Ariyasu, and Dr S. Muratsugu for generous help.Notes and references
- P. C. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2013, 52, 11980–11987 CrossRef CAS PubMed.
- S. J. Blansby and G. B. Ellinton, Acc. Chem. Res., 2003, 36, 255 CrossRef PubMed.
- A. E. Shilov and G. B. Shul’pin, Chem. Rev., 1997, 97, 2879–2932 CrossRef CAS PubMed.
- C. Copéret, Chem. Rev., 2010, 110, 656–680 CrossRef PubMed.
- G. B. Shul'pin, Dalton Trans., 2013, 42, 12794–12818 RSC.
- A. C. Lindhorst, S. Haslinger and F. E. Kuhn, Chem. Commun., 2015, 51, 17193–17212 RSC.
- D. S. Nesterov, O. V. Nesterova and A. J. L. Pombeiro, Coord. Chem. Rev., 2018, 355, 199–222 CrossRef CAS.
- M. Ravi, M. Ranocchiari and J. A. van Bokhoven, Angew. Chem., Int. Ed., 2017, 56, 16464–16483 CrossRef CAS PubMed.
- M. Bordeaux, A. Galarneau and J. Drone, Angew. Chem., Int. Ed., 2012, 51, 10712–10723 CrossRef CAS PubMed.
- T. J. Lawton and A. C. Rosenzweig, J. Am. Chem. Soc., 2016, 138, 9327–9340 CrossRef CAS PubMed.
- A. Trehoux, J.-P. Mahy and F. Avenier, Coord. Chem. Rev., 2016, 322, 142–158 CrossRef CAS.
- V. C. Wang, S. Maji, P. P. Chen, H. K. Lee, S. S. Yu and S. I. Chan, Chem. Rev., 2017, 117, 8574–8621 CrossRef CAS PubMed.
- K. S. Rabe, V. J. Gandubert, M. Spengler, M. Erkelenz and C. M. Niemeyer, Anal. Bioanal. Chem., 2008, 392, 1059–1073 CrossRef CAS PubMed.
- M. Sono, M. P. Roach, E. D. Coulter and J. H. Dawson, Chem. Rev., 1996, 96, 2841–2887 CrossRef CAS PubMed.
- A. R. McDonald and L. Que, Coord. Chem. Rev., 2013, 257, 414–428 CrossRef CAS.
- W. Nam, Acc. Chem. Res., 2007, 40, 522–531 CrossRef CAS PubMed.
- H. Fujii, Coord. Chem. Rev., 2002, 226, 51–60 CrossRef CAS.
- A. Gunay and K. H. Theopold, Chem. Rev., 2010, 110, 1060–1081 CrossRef CAS PubMed.
- A. B. Sorokin, Chem. Rev., 2013, 113, 8152–8191 CrossRef CAS PubMed.
- E. P. Talsi and K. P. Bryliakov, Coord. Chem. Rev., 2012, 256, 1418–1434 CrossRef CAS.
- C. W. Tse, T. W. Chow, Z. Guo, H. K. Lee, J. S. Huang and C. M. Che, Angew. Chem., Int. Ed., 2014, 53, 798–803 CrossRef CAS PubMed.
- G. V. Nizova, B. Krebs, G. Süss-Fink, S. Schindler, L. Westerheide, L. G. Cuervoc and G. B. Shul’pin, Tetrahedron, 2002, 58, 9231–9237 CrossRef CAS.
- S. I. Chan, Y. J. Lu, P. Nagababu, S. Maji, M. C. Hung, M. M. Lee, I. J. Hsu, P. D. Minh, J. C. Lai, K. Y. Ng, S. Ramalingam, S. S. Yu and M. K. Chan, Angew. Chem., Int. Ed., 2013, 52, 3731–3735 CrossRef CAS PubMed.
- A. B. Sorokin, E. V. Kudrik and D. Bouchu, Chem. Commun., 2008, 2562–2564 RSC.
- P. Afanasiev and A. B. Sorokin, Acc. Chem. Res., 2016, 49, 583–593 CrossRef CAS PubMed.
- L. X. Alvarez and A. B. Sorokin, J. Organomet. Chem., 2015, 793, 139–144 CrossRef CAS.
- E. V. Kudrik, P. Afanasiev, L. X. Alvarez, P. Dubourdeaux, M. Clemancey, J. M. Latour, G. Blondin, D. Bouchu, F. Albrieux, S. E. Nefedov and A. B. Sorokin, Nat. Chem., 2012, 4, 1024–1029 CrossRef CAS PubMed.
- M. G. Quesne, D. Senthilnathan, D. Singh, D. Kumar, P. Maldivi, A. B. Sorokin and S. P. de Visser, ACS Catal., 2016, 6, 2230–2243 CrossRef CAS.
- N. Mihara, Y. Yamada, H. Takaya, Y. Kitagawa, K. Igawa, K. Tomooka, H. Fujii and K. Tanaka, Chem. – Eur. J., 2019, 25, 3369–3375 CAS.
- L. C. Kao, A. C. Hutson and A. Sen, J. Am. Chem. Soc., 1991, 113, 700–701 CrossRef CAS.
- S. Morooka, C. Wakai, N. Matsubayasi and M. Nakahara, J. Phys. Chem. A, 2005, 109, 6610–6619 CrossRef CAS PubMed.
- V. M. Nosova, Y. A. Ustynyuk, L. G. Bruk, O. N. Temkin, A. V. Kisin and P. A. Storozhenko, Inorg. Chem., 2011, 50, 9300–9310 CrossRef CAS PubMed.
- S. S. H. Ho and J. Z. Yu, Anal. Chem., 2002, 74, 1232–1240 CrossRef CAS PubMed.
- X. Pang and A. C. Lewis, Anal. Methods, 2012, 4, 2013–2020 RSC.
- M. F. Zipplies, W. A. Lee and T. C. Bruice, J. Am. Chem. Soc., 1986, 108, 4433–4445 CrossRef CAS.
- Considering that TTN and MCN for methane oxidation started to decrease especially after 16 hours reaction, we decided to compare TTN and MCN values at 8 hours reaction.
- Y. Yamada, N. Mihara, S. Shibano, K. Sugimoto and K. Tanaka, J. Am. Chem. Soc., 2013, 135, 11505–11508 CrossRef CAS PubMed.
- N. Mihara, Y. Yamada, S. Akine, K. Sugimoto and K. Tanaka, Chem. Commun., 2017, 53, 2230–2232 RSC.
- K. Yamaguchi, K. Watanabe and I. Morishima, Inorg. Chem., 1992, 31, 156–157 CrossRef CAS.
- K. Yamaguchi, Y. Watanabe and I. Morishima, J. Chem. Soc., Chem. Commun., 1992, 1709–1710 RSC.
- K. Watanabe, in High-Valent Intermediates, ed. K. M. Kadish, K. M. Smith and R. Guilard, Elsevier, 2003, vol. 4, ch. 30 Search PubMed.
- S. Yokota and H. Fujii, J. Am. Chem. Soc., 2018, 140, 5127–5137 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nj02210d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |


