Dicationic ditelluride salts stabilized by N-heterocyclic carbene†
Abstract
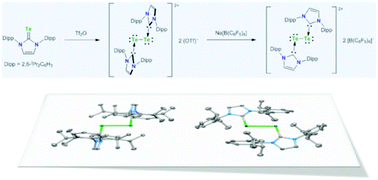
Treatment of 1,3-bis(2,6-diisopropylphenyl)imidazoline-2-tellone 1 with Tf2O in CH2Cl2 resulted in the formation of dicationic ditelluride salt 22+ 2 (OTf)− as air-stable green crystals in 88% yield. The anion exchange reaction of 22+ 2 (OTf)− with Na[B(C6F5)4] proceeded in CH3CN at room temperature giving the corresponding borate salt 22+ 2 [B(C6F5)4]− as blue crystals in 40% yield. The structures of these dicationic ditelluride derivatives were fully characterized on the basis of their NMR spectroscopic data and X-ray crystallography. In the crystalline state of 22+ 2 (OTf)−, two NHC ligands are entirely perpendicular to the central Te–Te bond with a Te–Te–C–N torsion angle of 89.93(19)°. In sharp contrast, X-ray analysis of 22+ 2 [B(C6F5)4]− revealed that two NHC ligands and the central Te–Te moiety are essentially coplanar with a Te–Te–C–N torsion angle of 7.99(17)°. In the DFT calculation for the model compound 32+, NBO orbitals around the Te–Te moiety in Ci symmetry consist of a Te–Te σ-bonding orbital, a Te–C σ-bonding orbital, a lone pair orbital on the Te atom (mainly s-character), and a lone pair orbital on the Te atom (pure p-character).
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...