A putative heme manganese(v)-oxo species in the C–H activation and epoxidation reactions in an aqueous buffer†‡
Abstract
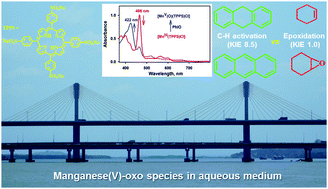
A water-soluble manganese(V)-oxo species 1 was generated in the reaction of [Mn(III)(TPPS)Cl] 2 (TPPS = 5,10,15,20-tetrakis(4-sulfonatophenyl)-21H,23H-porphine) and iodosylbenzene (PhIO) in a 2 : 1 aqueous buffer (pH = 10.4) : acetonitrile (CH3CN) mixture. The formula of the EPR silent species 1 is proposed as [Mn(V)(O)(TPPS)Cl] based on the Soret band (422 nm) and Q bands (520, 660 nm) in its UV-vis spectrum and its reaction with thioanisole, regenerating 2 and methyl phenyl sulfoxide. The reactivity of 1 was investigated in the C–H activation of alkyl hydrocarbons and epoxidation of cyclohexene. Based on the observation of the linear correlation of the logarithm of the second rate constant (log k2′) and the bond dissociation energy (BDE, kcal mol−1) of alkyl hydrocarbons along with a large kinetic isotope effect (KIE = 8.5) for xanthene vs. xanthene-d2, we propose H-atom abstraction as the rate determining step in the C–H activation reactions. On the other hand, in contrast to the C–H activation reaction, cyclohexene, which has a weak C–H bond (BDE = 82.5 kcal mol−1), undergoes an epoxidation reaction.


 Please wait while we load your content...
Please wait while we load your content...