Open Access Article
Open Access ArticleDo An(III) and Ln(III) ions form heteroleptic complexes with diglycolamide and hydrophilic BT(B)P ligands in solvent extraction systems? A spectroscopic and DFT study†
Irena
Herdzik-Koniecko
 a,
Christoph
Wagner
bc,
Michael
Trumm
b,
Udo
Müllich
b,
Bernd
Schimmelpfennig
b,
Jerzy
Narbutt
a,
Christoph
Wagner
bc,
Michael
Trumm
b,
Udo
Müllich
b,
Bernd
Schimmelpfennig
b,
Jerzy
Narbutt
 a,
Andreas
Geist
a,
Andreas
Geist
 *b and
Petra J.
Panak
bc
*b and
Petra J.
Panak
bc
aInstitute of Nuclear Chemistry and Technology (IChTJ), Dorodna 16, 03-195 Warsaw, Poland
bKarlsruhe Institute of Technology (KIT), Institute for Nuclear Waste Disposal (INE), P. O. Box 3640, 76021 Karlsruhe, Germany. E-mail: andreas.geist@kit.edu
cRuprecht-Karls-Universität Heidelberg, Institut für Physikalische Chemie, Im Neuenheimer Feld 234, 69120 Heidelberg, Germany
First published on 21st March 2019
Abstract
Time-Resolved Laser Fluorescence Spectroscopy (TRLFS) was used to study the complexation of Cm(III) and Eu(III) in solvent extraction systems consisting of an organic phase containing TODGA (tetra-n-octyl diglycolamide) as an extracting agent and an aqueous phase containing hydrophilic complexing agents, tetrasodium salts of 2,6-bis(5,6-di(3-sulphophenyl)-1,2,4-triazin-3-yl)pyridine, SO3-Ph-BTP4−, or 6,6′-bis(5,6-di(3-sulphophenyl)-1,2,4-triazin-3-yl)-2,2′-bipyridine, SO3-Ph-BTBP4−. Only one complex, homoleptic [Cm(TODGA)3](NO3)3, was found in the organic phase. No heteroleptic complexes of Cm(III) with TODGA and SO3-Ph-BTP4− have been detected. In contrast, in dilute aqueous HClO4 solutions containing TEDGA (a hydrophilic homologue of TODGA) and SO3-Ph-BTP4− ligands, seven different Cm(III) species were identified by their distinct emission spectra. Apart from the emission bands corresponding to the hydrated Cm3+ cation and to the complexes [Cm(SO3-Ph-BTP)]− and [Cm(TEDGA)n]3+ (n = 1–3), two further emission bands were ascribed to heteroleptic complexes [Cm(TEDGA)(SO3-Ph-BTP)]− and [Cm(TEDGA)2(SO3-Ph-BTP)]−. In a similar aqueous solution containing TEDGA and SO3-Ph-BTBP4−, only one heteroleptic complex was identified, [Cm(TEDGA)(SO3-Ph-BTBP)]−. In addition, quantum chemical optimisation of the structures of the postulated heteroleptic complexes was performed.
Introduction
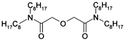
Long-term radiotoxicity and the heat load of irradiated nuclear fuels are mainly due to the presence of transuranium (TRU) elements, particularly plutonium and americium. Recycling the TRU elements from irradiated nuclear fuels would lead to a significant reduction of this hazard, and to improved utilization of natural resources since less uranium mining for the production of new nuclear fuels would be required, decreasing the environmental footprint of nuclear energy.1–3 Hence, strategies for the separation of the TRU elements from irradiated nuclear fuels and their fissioning (“burning”) in nuclear reactors are considered in many countries.4–6 The separation of plutonium is implemented in the PUREX process (Plutonium Uranium Reduction Extraction).7 Furthermore, also neptunium can be separated under similar conditions in an advanced version of the PUREX process.8,9 However, selective separation of the trivalent actinides, An(III), americium and curium, from the resulting PUREX raffinate, leaving the chemically similar lanthanide fission products, Ln(III), is extremely challenging.10 Nonetheless, this separation is extremely important since some lanthanide isotopes having large cross sections for neutron capture would significantly reduce the efficiency of fissioning actinides. Hence, highly selective ligands are required as extractants to achieve this separation.Alkylated N-donor ligands containing a 2,6-bis(5,6-dialkyl-1,2,4-triazin-3-yl)pyridine (BTP), 6,6′-bis(5,6-dialkyl-1,2,4-triazin-3-yl)-2,2′-bipyridine (BTBP) or 2,9-bis(5,6-dialkyl-1,2,4-triazin-3-yl)-1,10-phenanthroline (BTPhen) core structure have excellent selectivity for the extraction of An(III) over Ln(III) from nitric acid (see ref. 11–13 and the references therein). By exchanging the alkyl groups for sulfonated phenyl moieties, novel water-soluble N-donor ligands were synthesised (Scheme 1), easily dissociating into anions of −4 charge, even in acidic aqueous solutions. These complexing agents retain the BT(B)P selectivity for An(III)-over-Ln(III) complexation in the aqueous phase, offering new possibilities for the development of separation processes.14–21 The combination of co-extraction of An(III) and Ln(III) from the PUREX raffinate by using a lipophilic diglycolamide ligand TODGA (N,N,N′,N′-tetra-n-octyl-diglycolamide, Scheme 2), followed by selective stripping of An(III) using a hydrophilic BTP, 2,6-bis(5,6-di(3-sulphophenyl)-1,2,4-triazin-3-yl)pyridine, (SO3-Ph-BTP4−, Scheme 1 top) dissolved in the aqueous phase, results in large separation factors of An(III) over Ln(III), which is the basis of various separation processes.22–26
However, the experimental results related to the complexation of An(III) with these anionic N-donor ligands in the aqueous phase were rather difficult to interpret. Since Am(III) and Cm(III) prefer 9-fold coordination,27,28 their coordination with three tridentate SO3-Ph-BTP4− ligands was expected. Indeed, the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 M(III)-BTP complexes in solution both with hydrophobic BTP29–32 and with SO3-Ph-BTP4−
3 M(III)-BTP complexes in solution both with hydrophobic BTP29–32 and with SO3-Ph-BTP4−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15,33 had been proven spectroscopically. In contrast, slope analysis of Am(III) distribution data (i.e. a log–log plot of Am(III) distribution ratio vs. SO3-Ph-BTP4− concentration) in the TODGA extraction system yielded a value close to −2,14 suggesting the formation of the 1
15,33 had been proven spectroscopically. In contrast, slope analysis of Am(III) distribution data (i.e. a log–log plot of Am(III) distribution ratio vs. SO3-Ph-BTP4− concentration) in the TODGA extraction system yielded a value close to −2,14 suggesting the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex merely. A discrepancy between the results of the spectroscopic and solvent extraction studies was observed not only in the stoichiometries of the M(III)-SO3-Ph-BTP complexes but also in their stabilities. In a previous solvent extraction study on the complexation of Am(III) with SO3-Ph-BTP4− only two complexes, 1
2 complex merely. A discrepancy between the results of the spectroscopic and solvent extraction studies was observed not only in the stoichiometries of the M(III)-SO3-Ph-BTP complexes but also in their stabilities. In a previous solvent extraction study on the complexation of Am(III) with SO3-Ph-BTP4− only two complexes, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, were found,34 with conditional stability constants lower by more than an order of magnitude than the corresponding values determined spectroscopically for the corresponding complexes of Cm(III).15
2, were found,34 with conditional stability constants lower by more than an order of magnitude than the corresponding values determined spectroscopically for the corresponding complexes of Cm(III).15
Similar observations were made for the related tetradentate chelators SO3-Ph-BTBP and SO3-Ph-BTPhen (Scheme 1). The expected formation of Cm(III) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with these ligands was confirmed by spectroscopic studies in aqueous solutions.17,21 However, slope analysis of liquid–liquid extraction experiments in the systems SO3-Ph-BTBP/TODGA and SO3-Ph-BTPhen/TODGA yielded values of approximately −1.3 and −0.8, respectively,18,21 which implies the formation of only 1
2 complexes with these ligands was confirmed by spectroscopic studies in aqueous solutions.17,21 However, slope analysis of liquid–liquid extraction experiments in the systems SO3-Ph-BTBP/TODGA and SO3-Ph-BTPhen/TODGA yielded values of approximately −1.3 and −0.8, respectively,18,21 which implies the formation of only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of An(III) with these tetra-N-dentate ligands.
1 complexes of An(III) with these tetra-N-dentate ligands.
These discrepancies show that the model of the extraction processes of An(III) ions, based on the presence of only their homoleptic complexes (with TODGA in the organic phase and with SO3-Ph-(BTP/BTBP/BTPhen) in the aqueous phase) may be incorrect. A hypothesis which explains these discrepancies suggests the formation of heteroleptic complexes of An(III) with TODGA and SO3-Ph-BTP4−, e.g. [Am(SO3-Ph-BTP)(TODGA)2]−, which could be extracted in the organic phase e.g. as ion pairs with the protonated extractant, TODGA·H+. The formation of heteroleptic complexes to explain the behaviour of a similar solvent extraction system has been suggested earlier.35
This assumption requires careful investigation of the equilibria in each phase of solvent extraction systems containing two competing ligands – lipophilic and hydrophilic.
The aim of the present work was to find evidence for (or against) the existence of these hypothetical heteroleptic complexes, e.g. [Cm(SO3-Ph-BTP)(TODGA)2]−, in the solvent extraction systems containing the competing lipophilic and hydrophilic ligands: TODGA and SO3-Ph-BTP (L), respectively. If such complexes really existed and their concentration in the organic phase increased with the concentration of the hydrophilic ligand, the slope of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D vs. log[L4−] in the extraction experiments would change from the expected value of −3 to −2 as observed experimentally.14 Evidence had been found for the existence of such heteroleptic complexes in similar solvent extraction systems containing two competing neutral chelating ligands, lipophilic DMDOHEMA and one of the hydrophilic DGA derivatives (TEDGA, Me-TEDGA or Me2-TEDGA, Scheme 3). TRLFS measurements confirmed the exclusive existence of [Cm(DGA)3]3+ complexes in the aqueous phase. In the organic phase both the homoleptic [Cm(DMDOHEMA)n](NO3)3 and heteroleptic [Cm(DGA)x(DMDOHEMA)y](NO3)3 complexes were detected.36
D vs. log[L4−] in the extraction experiments would change from the expected value of −3 to −2 as observed experimentally.14 Evidence had been found for the existence of such heteroleptic complexes in similar solvent extraction systems containing two competing neutral chelating ligands, lipophilic DMDOHEMA and one of the hydrophilic DGA derivatives (TEDGA, Me-TEDGA or Me2-TEDGA, Scheme 3). TRLFS measurements confirmed the exclusive existence of [Cm(DGA)3]3+ complexes in the aqueous phase. In the organic phase both the homoleptic [Cm(DMDOHEMA)n](NO3)3 and heteroleptic [Cm(DGA)x(DMDOHEMA)y](NO3)3 complexes were detected.36
In the present work, time resolved laser fluorescence spectroscopy (TRLFS) studies were performed on (a) the complexation of Cm(III) in the solvent extraction system with TODGA and SO3-Ph-BTP4− and (b) the complexation of Cm(III) and Eu(III) in aqueous solutions with N,N,N′,N′-tetraethyl diglycolamide (TEDGA, Scheme 3) and SO3-Ph-BTP4− or SO3-Ph-BTBP4−.
The experimental results are supported by quantum chemical optimisation of the structures of postulated heteroleptic complexes.
Experimental
Chemicals
For the TRLFS studies a 2.1 × 10−5 mol L−1 Cm(ClO4)3 solution with an isotopic mass distribution of 89.7% Cm-248, 9.4% Cm-246, ≤1% Cm-243, Cm-244, Cm-245 and Cm-247 was used. SO3-Ph-BTP, SO3-Ph-BTBP and TODGA were prepared according to literature procedures.14,37,38 TEDGA was supplied by Technocomm Ltd, Edinburgh, UK, and used as received.Preparation of TRLFS samples
Stock solutions (10−2 mol L−1) of N-donor ligands were prepared by dissolving 9.49 mg of SO3-Ph-BTP or 10.26 mg of SO3-Ph-BTBP (tetrasodium salts) in 1 mL of 10−3 mol L−1 HClO4. Using suitable dilutions of the stock solutions the SO3-Ph-BTP/BTBP concentrations were adjusted to 5 × 10−6 mol L−1 and 4 × 10−7 mol L−1, respectively. A stock solution of 1 mol L−1 TEDGA was prepared by dissolving 244 mg of TEDGA in 1 mL of 10−3 mol L−1 HClO4, and the solutions used for titration experiments were prepared by subsequent dilutions.Titration experiments were performed by adding 4.7 μL of Cm(III) stock solution to 995 μL of 10−3 mol L−1 HClO4, resulting in an initial Cm(III) concentration of 10−7 mol L−1. After the addition of SO3-Ph-BTP or SO3-Ph-BTBP, the samples were shaken and equilibrated for 15 minutes before recording the emission spectrum. The TEDGA concentration was increased by stepwise addition of TEDGA solution. After each addition, an emission spectrum was recorded.
Solvent extraction experiments were performed by shaking each 0.5 mL organic (0.2 mol L−1 TODGA + 5 vol% octanol in TPH (“hydrogenated tetrapropylene”, a kerosene diluent)) and aqueous (10−7 mol L−1 Cm(III), 20 mmol L−1 SO3-Ph-BTP in 0.7 mol L−1 HNO3) phases using a vortex shaker (40 Hz) for 15 min, sufficient to attain equilibrium. The phases were separated by centrifugation and emission spectra were recorded.
Determination of SO3-Ph-BTP in organic phases
Transfer of SO3-Ph-BTP to the organic phase was determined spectrophotometrically by stripping SO3-Ph-BTP back to the aqueous phase and measuring the absorbance of the Fe(II)-SO-Ph-BTP complex on a Cary 6000i UV-vis spectrophotometer using cuvettes with a path length of 10 mm.Solutions containing (1–20) × 10−6 mol L−1 SO3-Ph-BTP, 3 × 10−4 mol L−1 Fe(NO3)3, 0.015 mol L−1 HNO3 and 0.1 mol L−1 hydroxylammonium chloride in 1 mol L−1 acetate buffer (pH = 4.5) were used to establish a calibration curve.
Since the formation of heteroleptic complexes requires the presence of metal ion complexes in the organic phase, transfer of SO3-Ph-BTP to the organic phase was determined (a) in the absence and (b) in the presence of Eu(III):
(a) Equal volumes of organic (0.2 mol L−1 TODGA + 5 vol% octanol in TPH) and aqueous (20 mmol L−1 SO3-Ph-BTP in 0.5 mol L−1 HNO3) solutions were mixed using a vortex shaker (40 Hz) for 15 min and the phases were separated after centrifugation. An aliquot of the organic phase was shaken with water (A/O = 3) to strip SO3-Ph-BTP4−. To this aqueous phase, solutions containing Fe(NO3)3, hydroxylammonium chloride and acetate buffer (at concentrations as used to establish the calibration curve) were added. The absorption spectrum was measured.
(b) Equal volumes of organic (0.2 mol L−1 TODGA + 5 vol% octanol in TPH) and aqueous (20 mmol L−1 SO3-Ph-BTP + 30 mmol L−1 Eu(NO3)3 in 0.5 mol L−1 HNO3) solutions were mixed using a vortex shaker (40 Hz) for 15 min and the phases were separated after centrifugation. An aliquot of the organic phase was shaken with water (A/O = 3) and with a solution of a 0.5 mol L−1 ammonium glycolate buffer, pH = 4 (A/O = 3) to strip SO3-Ph-BTP4−. The second stripping step was necessary since Eu(III) was not completely stripped in the first step. These aqueous phases were further processed as described above.
TRLFS setup
The TRLFS (Time-Resolved Laser Fluorescence Spectroscopy) setup consisted of a Nd:YAG laser pumped (Continuum Surelite Laser) dye laser system (NARROWscan D-R Dye Laser) with a repetition rate of 10 Hz. An ICCD camera with an integrated delay controller (iStar, Andor) was used to record the fluorescence signal after spectral decomposition using a Shamrock 303i spectrograph. For the excitation of Cm(III), a wavelength of 396.6 nm was applied. Scattering light and short-lived fluorescence of organic compounds were discriminated by a delay time of 1 μs between the excitation of Cm(III) and the measurement of the fluorescence signal. For the measurement of fluorescence lifetimes the delay time between excitation and recording of the fluorescence signal was increased stepwise in the range of 0–2100 μs.Quantum chemistry
Quantum chemical optimisation of the structures of relevant heteroleptic complexes with TEDGA allows for a detailed view of their structural properties. Accordingly, we used the density functional theory (DFT) method to optimise the structures of [Cm(SO3-Ph-BTP)(TEDGA)n(H2O)6−3n]− (n = 0, 1, 2), [Cm(SO3-Ph-BTBP)(TEDGA)n(H2O)5−3n]− (n = 0, 1) and 10-fold coordinated [Cm(SO3-Ph-BTBP)(TEDGA)2]−. In all calculations the BH-LYP functional39 as implemented in the TURBOMOLE software package40 was used, following the protocol of a recent study.41 The Cm(III) ion is described by the effective core potential (ECP60MWB42) and corresponding basis sets of triple zeta quality. All other atoms are described by the standard def-TZVP basis set.43Results and discussion
Part I: heteroleptic complexes in the organic phase by extraction experiments
The formation of heteroleptic complexes in the organic phase by extraction experiments would explain the aforementioned discrepancy between the results of slope analysis and spectroscopic studies. Spectroscopic techniques were applied to probe their presence in the systems studied.The exact agreement of the Cm(III) emission spectra of both the samples from the solvent extraction experiment containing SO3-Ph-BTP and TODGA with those from the reference experiments indicates the exclusive formation of the [Cm(SO3-Ph-BTP)3]9− complex in the aqueous phase and the [Cm(TODGA)3](NO3)3 complex in the organic phase. Thus the amount of potentially formed heteroleptic complexes in the organic phase must be very low, if any.
To determine whether heteroleptic complexes were formed in the organic phase, even in small amounts, direct excitation measurements were applied using Eu(III) as a substitute. An extraction experiment was performed using an aqueous phase containing initially 30 mmol L−1 Eu(III) and 20 mmol L−1 SO3-Ph-BTP in 0.5 mol L−1 nitric acid and an organic phase containing 0.2 mol L−1 TODGA + 5 vol% octanol in TPH. After shaking and phase separation, the emission spectra resulting from the 5D0 → 7F2 transition of Eu(III) in the organic phase were measured as a function of the excitation wavelength. These emission spectra are shown in Fig. 2, as well as the excitation spectrum of the Eu(III) 7F0 band. The excitation spectrum of a sample containing exclusively [Eu(TODGA)3](NO3)3 is also shown for comparison.
The normalised emission spectra of the 5D0 → 7F2 transition display the characteristic splitting of the [Eu(TODGA)3](NO3)3 complex44 and do not change with altering the excitation wavelength (Fig. 2, top). Furthermore, the excitation spectrum of the 5D0 → 7F2 transition shows only one emission band corresponding to the exclusive formation of [Eu(TODGA)3](NO3)3 in the organic phase (Fig. 2, bottom).
In a preliminary experiment, an aqueous sample containing 20 mmol L−1 SO3-Ph-BTP in 0.5 mol L−1 HNO3 was mixed with an organic phase consisting of 0.2 mol L−1 TODGA + 5 vol% 1-octanol in TPH (A/O = 1). The organic sample was shaken with water (A/O = 3) to strip SO3-Ph-BTP4−. An absorption spectrum of this aqueous phase was recorded. No absorption signal of the Fe(II)-SO3-Ph-BTP complex was detected, i.e. its concentration was below the detection limit of 10−6 mol L−1. With the A/O ratio of 3 used to strip the organic phase, the concentration of SO3-Ph-BTP transferred to the organic phase was below 3 × 10−6 mol L−1.
A further experiment was performed, mixing an organic phase (0.2 mol L−1 TODGA + 5 vol% 1-octanol in kerosene) with an aqueous phase containing Eu(III) (20 mmol L−1 SO3-Ph-BTP + 30 mmol L−1 Eu(NO3)3 in 0.5 mol L−1 HNO3), A/O = 1. The organic phase was shaken with water (A/O = 3) and then with 0.5 mol L−1 NH4-glycolate (pH =4; A/O = 3). The SO3-Ph-BTP concentrations in the stripping solutions were determined, resulting in an organic phase SO3-Ph-BTP concentration of 5.5 × 10−5 mol L−1.
Under these experimental conditions, Eu(III) was almost completely extracted to the organic phase. Assuming the formation of a heteroleptic Eu(III) complex with one SO3-Ph-BTP molecule, its concentration in the organic phase would also be 5.5 × 10−5 mol L−1, i.e. 0.2% relative to the [Eu(TODGA)3](NO3)3 complex. Such a small fraction is expected to remain undetected in the emission spectra.
Part II: heteroleptic complexes in monophasic aqueous solutions
Further research was aimed at clarifying whether heteroleptic complexes of Cm(III) with the hydrophilic ligands SO3-Ph-BTP4− or SO3-Ph-BTBP4− and TEDGA – a water-soluble homologue of TODGA – would form in aqueous solutions. | ||
| Fig. 3 Normalised emission spectra of Cm(III) in 10−3 mol L−1 HClO4 solution at increasing TEDGA concentration in the presence of 5 × 10−6 mol L−1 SO3-Ph-BTP. | ||
Prior to the first addition of TEDGA, the emission spectrum displays two emission bands at 593.9 nm and 602.3 nm. These emission bands correspond to the aqua ion of Cm(III) and to the [Cm(SO3-Ph-BTP)]− complex, and are in excellent agreement with the literature.15
With increasing TEDGA concentration, five additional emission bands are observed at 598.3 nm, 603.3 nm, 606.9 nm, 609.2 nm and 612.5 nm. The emission bands at 598.3 nm and 603.3 nm are present in the range of TEDGA concentrations from 5 × 10−6 mol L−1 to 3.6 × 10−2 mol L−1, displaying only low intensities. These bands are attributed to the [Cm(TEDGA)]3+ and [Cm(TEDGA)2]3+ complexes,36 and are in good agreement with the spectra observed for the chemically related lipophilic extractant TODGA.44 The emission band at 609.2 nm starts to form at TEDGA concentrations ≥2 × 10−3 mol L−1 and is the dominating emission band at TEDGA concentrations ≥10−2 mol L−1. This emission band is attributed to the [Cm(TEDGA)3]3+ complex in comparison with the literature.36 This is in excellent agreement with the Cm(III) emission spectrum of the [Cm(TODGA)3]3+ complex.44
Hence, the emission bands at 606.9 nm and 612.5 nm remain unidentified. The emission band at 606.9 nm is formed at TEDGA concentrations ≥5 × 10−6 mol L−1. Formation of the second unidentified emission band at 612.5 nm begins at TEDGA concentrations ≥3.5 × 10−5 mol L−1.
To investigate the composition of the complexes corresponding to the unidentified emission bands the pure component spectra of all species were obtained from the spectra of the titration experiment by peak deconvolution (Fig. 4). Applying the pure component spectra, the fractions of the present complex species were determined as a function of the TEDGA concentration.
 | ||
| Fig. 4 Pure component spectra of the Cm(III) complexes with SO3-Ph-BTP4−, and TEDGA and the heteroleptic complexes. | ||
Since the spectra of all the complex species containing solely SO3-Ph-BTP or TEDGA are known, the new emission bands at 606.9 nm and 612.5 nm may be attributed to the heteroleptic complexes containing SO3-Ph-BTP4− and TEDGA. Hence, the following equilibrium was assumed:
| [Cm(SO3-Ph-BTP)(TEDGA)n−1]− + TEDGA ⇄ [Cm(SO3-Ph-BTP)(TEDGA)n]− |
The Cm(III) species distribution in aqueous solution titrated with TEDGA in the presence of SO3-Ph-BTP is shown in Fig. 6. Under the experimental conditions (10−3 mol L−1 HClO4, 5.0 × 10−6 mol L−1 SO3-Ph-BTP), the first heteroleptic complex, [Cm(SO3-Ph-BTP)(TEDGA)]− has a maximum fraction of approximately 30% at 1.9 × 10−4 mol L−1 TEDGA. The second heteroleptic complex, [Cm(SO3-Ph-BTP)(TEDGA)2]− is the dominating species for TEDGA concentrations between 3.5 × 10−4 mol L−1 and 3.0 × 10−2 mol L−1, with a maximum fraction of approximately 70% at a TEDGA concentration of 4.6 × 10−3 mol L−1.
The fluorescence lifetime of the [Cm(SO3-Ph-BTP)(TEDGA)2]− complex is 333 μs. This is significantly longer than the fluorescence lifetime of [Cm(SO3-Ph-BTP)3]9− (τ = 202 μs)15 and shorter than the fluorescence lifetime of the [Cm(TODGA)3](NO3)3 complex (τ = 517 μs).44 The comparatively short fluorescence lifetime of [Cm(SO3-Ph-BTP)3]9− is induced by quenching effects of N-donor ligands in complexes with Cm(III).45,46 Since diglycolamide ligands do not exhibit fluorescence quenching effects,44 quenching effects in a heteroleptic complex should be less pronounced than those in [Cm(SO3-Ph-BTP)3]9−, but more than those in [Cm(TODGA)3](NO3)3. Thus, the observed fluorescence lifetime of [Cm(SO3-Ph-BTP)(TEDGA)2]− is in agreement with inner-sphere coordination of TEDGA molecules, confirming the results obtained by slope analysis.
 | ||
| Fig. 7 Normalised emission spectra of Cm(III) in 10−3 mol L−1 HClO4 solution at increasing TEDGA concentration in the presence of 4 × 10−7 mol L−1 SO3-Ph-BTBP. | ||
The initial Cm(III) emission spectrum (in the absence of TEDGA) displays three emission bands at 593.9 nm, 606.2 nm and 618.9 nm, corresponding to the Cm(III) aqua ion and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes,17 [Cm(SO3-Ph-BTBP)]− and [Cm(SO3-Ph-BTBP)2]5−. Upon addition of TEDGA, four additional spectra are observed. Three of them (displaying peak maxima at 598.3 nm, 603.3 nm and 609.2 nm) are assigned to the [Cm(TEDGA)n]3+ (n = 1–3) complexes.36 Finally, a peak with a maximum at 612 nm is observed. Following the reasoning explained for the TEDGA/SO3-Ph-BTP heteroleptic complexes, this emission band is assigned to the heteroleptic complex, [Cm(SO3-Ph-BTBP)(TEDGA)]−.
2 complexes,17 [Cm(SO3-Ph-BTBP)]− and [Cm(SO3-Ph-BTBP)2]5−. Upon addition of TEDGA, four additional spectra are observed. Three of them (displaying peak maxima at 598.3 nm, 603.3 nm and 609.2 nm) are assigned to the [Cm(TEDGA)n]3+ (n = 1–3) complexes.36 Finally, a peak with a maximum at 612 nm is observed. Following the reasoning explained for the TEDGA/SO3-Ph-BTP heteroleptic complexes, this emission band is assigned to the heteroleptic complex, [Cm(SO3-Ph-BTBP)(TEDGA)]−.
Pure component spectra obtained by peak deconvolution are given in Fig. 8.
 | ||
| Fig. 8 Pure component spectra of the Cm(III) complexes with SO3-Ph-BTBP, and TEDGA and heteroleptic complexes. | ||
The double logarithmic plot of the molar concentration ratio of c[Cm(SO3-Ph-BTBP)(TEDGA)−] to c[Cm(SO3-Ph-BTBP)−] yielded a value of 1.1 for the addition of TEDGA to the Cm(SO3-Ph-BTBP)− complex, evidencing the formation of the heteroleptic complex, [Cm(SO3-Ph-BTBP)(TEDGA)]−.
Applying the pure component spectra (Fig. 8), the fractions of the complex species were determined as a function of the TEDGA concentration. The distribution of the Cm(III) species as a function of the TEDGA concentration in the presence of SO3-Ph-BTBP is shown in Fig. 9. Under the experimental conditions (10−3 mol L−1 HClO4, 4 × 10−7 mol L−1 SO3-Ph-BTBP), the heteroleptic complex, [Cm(SO3-Ph-BTBP)(TEDGA)]− is present over a wide range of TEDGA concentrations, (10−5–10−1) mol L−1. It has a maximum fraction of approximately 25% at 8 × 10−4 mol L−1 TEDGA.
 | ||
| Fig. 9 Cm(III) species distribution in 10−3 mol L−1 HClO4 as a function of the TEDGA concentration in the presence of 4 × 10−7 mol L−1 SO3-Ph-BTBP. | ||
The fluorescence lifetime of Cm(SO3-Ph-BTBP)(TEDGA)− was not determined because the relative intensity of its emission band was too low and it overlapped with other emission bands.
Structural information
Supporting the findings from the experiments, all optimised heteroleptic complexes were found to be true minima on the potential surface. The bond distances are similar to those in the corresponding lipophilic BT(B)P derivatives,41 except for those with the pyridine nitrogen atom, Npyr. The repulsion of the negatively charged sulfonate groups leads to an increased flexion of the ligand, increasing the Cm–Npyr bond length (Table 1).| Cm–Npyr | Cm–Ntri | Cm–Oeth | Cm–Oamid | |
|---|---|---|---|---|
| [Cm(SO3-Ph-BTP)(H2O)6]− | 2.85 | 2.63 | — | — |
| [Cm(SO3-Ph-BTP)(TEDGA)(H2O)3]− | 2.73 | 2.58 | 2.53 | 2.39 |
| [Cm(SO3-Ph-BTP)(TEDGA)2]− | 2.69 | 2.61 | 2.59 | 2.43 |
In particular, the bonds with the Npyr and Ntri atoms in the [Cm(SO3-Ph-BTBP)(TEDGA)2]− complex are elongated to compensate for the increased coordination number of the Cm(III) ion. However, this change in the coordination mode is unlikely to prevail in solution due to the large Cm–Npyr bond length. This is in agreement with the results of TRLFS where the [Cm(SO3-Ph-BTBP)(TEDGA)2]− complex was not observed (Table 2).
| Cm–Npyr | Cm–Ntri | Cm–Oeth | Cm–Oamid | |
|---|---|---|---|---|
| [Cm(SO3-Ph-BTBP)(H2O)5]− | 2.76 | 2.55 | — | — |
| [Cm(SO3-Ph-BTBP)(TEDGA)(H2O)2]− | 2.71 | 2.53 | 2.56 | 2.44 |
| [Cm(SO3-Ph-BTBP)(TEDGA)2]− | 3.01 | 2.78 | 2.60 | 2.46 |
Conclusions
Solvent extraction systems combining a non-selective extracting agent, TODGA, and a water soluble complexing agent, SO3-Ph-BTP, SO3-Ph-BTBP, or SO3-Ph-BTPhen, have been developed and tested to separate An(III) from Ln(III). Results from solvent extraction experiments cannot be explained by simple models assuming the formation of only TODGA complexes in the organic phase and SO3-Ph-BTP/SO3-Ph-BTBP/SO3-Ph-BTPhen complexes in the aqueous phase. A spectroscopic investigation was performed to understand whether the formation of heteroleptic complexes in the organic phase explains this discrepancy.The speciation in organic and aqueous phase samples from Cm(III) extraction experiments with TODGA and SO3-Ph-BTP or SO3-Ph-BTBP was investigated by TRLFS. Only homoleptic complexes, [Cm(TODGA)3](NO3)3 and [Cm(SO3-Ph-BTP)3]9− or [Cm(SO3-Ph-BTBP)2]5−, were detected in the organic and aqueous phases, respectively.
However, the presence of low concentrations of SO3-Ph-BTP in the organic phase by an extraction experiment employing a rather large concentration of Eu(III) was detected photometrically. Since in the absence of Eu(III), no SO3-Ph-BTP was detected in the organic phase, this result is indirect evidence for the formation of a heteroleptic complex. Its concentration was estimated to be as low as 0.2% relative to that of the [Eu(TODGA)3](NO3)3 complex, which explains why it went undetected by TRLFS.
Finally, aqueous monophasic systems containing TEDGA and SO3-Ph-BTP or SO3-Ph-BTBP were studied. With SO3-Ph-BTP, two heteroleptic complexes, [Cm(SO3-Ph-BTP)(TEDGA)]− and [Cm(SO3-Ph-BTP)(TEDGA)2]−, were identified. The heteroleptic complex [Cm(SO3-Ph-BTBP)(TEDGA)]− was identified in the system containing SO3-Ph-BTBP. Optimised structures of these complexes were calculated by DFT.
While this study is the first to support the existence of heteroleptic complexes in solvent extraction systems combining heterocyclic N-donor ligands and TODGA, its results may be of more general relevance: for example, in the case of another water-soluble N-donor ligand, 2,6-bis(1H-1,2,3-triazol-4-yl) pyridine, similar discrepancies between the results of solvent extraction47 and spectroscopic studies48 were observed.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support for this research was provided by the European Commission via the following projects: SACSESS (contract No. FP7-Fission-2012-323282), GENIORS (Horizon 2020 grant agreement No. 755171) and TALISMAN (contract No. FP7-Fission-2012-323300).Notes and references
- C. Poinssot, S. Bourg, N. Ouvrier, N. Combernoux, C. Rostaing, M. Vargas-Gonzalez and J. Bruno, Energy, 2014, 69, 199 CrossRef.
- C. Poinssot, S. Bourg and B. Boullis, Prog. Nucl. Energy, 2016, 92, 234 CrossRef CAS.
- C. Poinssot, S. Bourg, S. Grandjean and B. Boullis, Procedia Chem., 2016, 21, 536 CrossRef.
- OECD-NEA, Advanced Nuclear Fuel Cycles and Radioactive Waste Management, NEA No. 5990, OECD, Nuclear Energy Agency (NEA), Paris, 2006.
- OECD-NEA, Potential Benefits and Impacts of Advanced Nuclear Fuel Cycles with Actinide Partitioning and Transmutation, NEA No. 6894, OECD, Nuclear Energy Agency (NEA), Paris, 2011.
- C. Poinssot, C. Rostaing, S. Greandjean and B. Boullis, Procedia Chem., 2012, 7, 349 CrossRef CAS.
- W. B. Lanham and T. C. Runion, PUREX process for plutonium and uranium recovery, USAEC report ORNL-479, Oak Ridge National Laboratory, USA, 1949.
- B. Dinh, P. Moisy, P. Baron, J.-N. Calor, D. Espinoux, B. Lorrain and M. Benchikouhne-Ranchoux, Proc. Internat. Solvent Extr. Conf. (ISEC 2008), Tucson, Arizona, USA, 15–19 September, 2008.
- R. J. Taylor, C. R. Gregson, M. J. Carrott, C. Mason and M. J. Sarsfield, Solvent Extr. Ion Exch., 2013, 31, 442 CrossRef CAS.
- G. T. Seaborg, Radiochim. Acta, 1993, 61, 115 CAS.
- C. Ekberg, A. Fermvik, T. Retegan, G. Skarnemark, M. R. S. Foreman, M. J. Hudson, S. Englund and M. Nilsson, Radiochim. Acta, 2008, 96, 225 CAS.
- F. W. Lewis, M. J. Hudson and L. M. Harwood, Synlett, 2011, 2609 CAS.
- P. J. Panak and A. Geist, Chem. Rev., 2013, 113, 1199–1236 CrossRef CAS PubMed.
- A. Geist, U. Müllich, D. Magnusson, P. Kaden, G. Modolo, A. Wilden and T. Zevaco, Solvent Extr. Ion Exch., 2012, 30, 433 CrossRef CAS.
- C. M. Ruff, U. Müllich, A. Geist and P. J. Panak, Dalton Trans., 2012, 41, 14594 RSC.
- H. Galán, D. Munzel, A. Núñez, U. Müllich, J. Cobos and A. Geist, Proc. Internat. Solvent Extr. Conf. (ISEC 2014), Würzburg, Germany, 7–11 September, 2014.
- C. Wagner, U. Müllich, A. Geist and P. J. Panak, Dalton Trans., 2015, 44, 17143 RSC.
- C. Wagner, U. Müllich, A. Geist and P. J. Panak, Solvent Extr. Ion Exch., 2016, 34, 103 CrossRef CAS.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, A. Geist, V. N. Kozhevnikov, P. Distler and J. John, Chem. Sci., 2015, 6, 4812 RSC.
- P. Kaufholz, F. Sadowski, A. Wilden, G. Modolo, F. W. Lewis, A. W. Smith and L. M. Harwood, Nukleonika, 2015, 60, 815 Search PubMed.
- P. Kaufholz, G. Modolo, A. Wilden, F. Sadowski, D. Bosbach, C. Wagner, A. Geist, P. J. Panak, F. W. Lewis and L. M. Harwood, Solvent Extr. Ion Exch., 2016, 34, 126 CrossRef CAS.
- A. Wilden, G. Modolo, P. Kaufholz, F. Sadowski, S. Lange, M. Sypula, D. Magnusson, U. Müllich, A. Geist and D. Bosbach, Solvent Extr. Ion Exch., 2015, 33, 91 CrossRef CAS.
- M. Carrott, K. Bell, J. Brown, A. Geist, C. Gregson, X. Hérès, C. Maher, R. Malmbeck, C. Mason, G. Modolo, U. Müllich, M. Sarsfield, A. Wilden and R. Taylor, Solvent Extr. Ion Exch., 2014, 32, 447 CrossRef CAS.
- M. Carrott, A. Geist, X. Hérès, S. Lange, R. Malmbeck, M. Miguirditchian, G. Modolo, A. Wilden and R. Taylor, Hydrometallurgy, 2015, 152, 139 CrossRef CAS.
- M. Carrott, C. Maher, C. Mason, M. Sarsfield and R. Taylor, Sep. Sci. Technol., 2016, 51, 2198 CrossRef CAS.
- R. Malmbeck, D. Magnusson, S. Bourg, M. Carrott, A. Geist, X. Hérès, M. Miguirditchian, G. Modolo, U. Müllich, C. Sorel, R. Taylor and A. Wilden, Radiochim. Acta, 2019 Search PubMed , submitted.
- P. Lindqvist-Reis, R. Klenze, G. Schubert and T. Fanghänel, J. Phys. Chem. B, 2005, 109, 3077 CrossRef CAS PubMed.
- P. Lindqvist-Reis, C. Walther, R. Klenze and N. M. Edelstein, J. Phys. Chem. C, 2009, 113, 449 CrossRef CAS.
- M. A. Denecke, A. Rossberg, P. J. Panak, M. Weigl, B. Schimmelpfennig and A. Geist, Inorg. Chem., 2005, 44, 8418 CrossRef CAS PubMed.
- M. A. Denecke, P. J. Panak, F. Burdet, M. Weigl, A. Geist, R. Klenze, M. Mazzanti and K. Gompper, C. R. Chim, 2007, 10, 872 CrossRef CAS.
- N. L. Banik, B. Schimmelpfennig, C. M. Marquardt, B. Brendebach, A. Geist and M. A. Denecke, Dalton Trans., 2010, 39, 5117 RSC.
- N. L. Banik, M. A. Denecke, A. Geist, G. Modolo, P. J. Panak and J. Rothe, Inorg. Chem. Commun., 2013, 29, 172 CrossRef CAS.
- C. M. Ruff, Spektroskopische und thermodynamische Untersuchung der Komplexierung von Cm(III) und Eu(III) mit hydrophilen Bis-Triazinylpyridinen, Dissertation, Universität Heidelberg, Germany, 2013.
- Ł. Steczek, M. Rejnis, J. Narbutt, M.-C. Charbonnel and P. Moisy, J. Radioanal. Nucl. Chem., 2016, 309, 891 Search PubMed.
- S. Chapron, C. Marie, V. Pacary, M. T. Duchesne, G. Arrachart, S. Pellet-Rostaing and M. Miguirditchian, Procedia Chem., 2016, 21, 133 CrossRef.
- L. Klaß, A. Wilden, F. Sadowski, C. Wagner, A. Geist, P. J. Panak, I. Herdzik-Koniecko, J. Narbutt and G. Modolo, Solvent Extr. Ion Exch., 2018 Search PubMed , submitted.
- G. L. Traister and A. A. Schilt, Anal. Chem., 1976, 48, 1216 CrossRef CAS PubMed.
- Y. Sasaki and G. R. Choppin, Anal. Sci., 1996, 12, 225 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 1372 CrossRef CAS.
- Turbomole, http://www.turbomole.com.
- M. Trumm and B. Schimmelpfennig, Mol. Phys., 2016, 114, 876 CrossRef CAS.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, J. Chem. Phys., 1994, 100, 7535 CrossRef.
- A. Schäfer, C. Huber and R. Ahlrichs, J. Chem. Phys., 1994, 100, 5829 CrossRef.
- A. Wilden, G. Modolo, S. Lange, F. Sadowski, B. B. Beele, A. Skerencak-Frech, P. J. Panak, M. Iqbal, W. Verboom, A. Geist and D. Bosbach, Solvent Extr. Ion Exch., 2014, 32, 119 CrossRef CAS.
- S. Trumm, G. Lieser, M. R. S. Foreman, P. J. Panak, A. Geist and T. Fanghänel, Dalton Trans., 2010, 39, 923 RSC.
- M. Trumm, C. Wagner, B. Schimmelpfennig, A. Geist and P. J. Panak, Dalton Trans., 2016, 45, 12308 RSC.
- E. Macerata, E. Mossini, S. Scaravaggi, M. Mariani, A. Mele, W. Panzeri, N. Boubals, L. Berthon, M.-C. Charbonnel, F. Sansone, A. Arduini and A. Casnati, J. Am. Chem. Soc., 2016, 138, 7232 CrossRef CAS PubMed.
- C. Wagner, E. Mossini, E. Macerata, M. Mariani, A. Arduini, A. Casnati, A. Geist and P. J. Panak, Inorg. Chem., 2017, 56, 2135 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nj00651f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |