Efficient syntheses and antimicrobial activities of new thiophene containing pyranone and quinolinone derivatives using manganese(iii) acetate: the effect of thiophene on ring closure–opening reactions†
Abstract
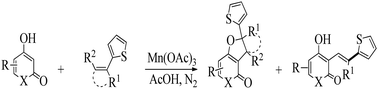
The syntheses of a new series of pyranones, namely, fused pyranones and quinoline-based dihydrofurans, accompanied by 3-alkenyl-substituted structures were investigated. The products were regioselectively formed by Mn(III)-mediated oxidation at elevated temperature in excellent yields. The effects of the thiophene group and reaction temperature and time on product distributions were investigated. The structures of the synthesized compounds were determined on the basis of spectroscopic (IR, 1H NMR, 13C NMR, COSY, HSQC, HMBC and elemental analysis) and X-ray crystallographic data. In addition, the in vitro antimicrobial activities of some synthesized dihydrofurans were tested against G (+) and G (−) bacteria using the disc diffusion method. The results indicated that the compounds containing the thiophene group showed a better antimicrobial effect than some commonly used antibiotics.


 Please wait while we load your content...
Please wait while we load your content...