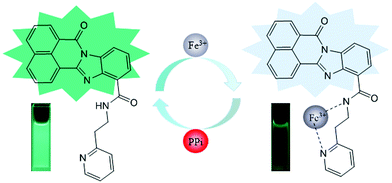
A new “ON–OFF–ON” fluorescent probe for sequential detection of Fe3+ and PPi based on 2-pyridin-2-ylethanamine and benzimidazo [2,1-a]benz[de]isoquinoline-7-one-12-carboxylic acid†
Yuankang
Xu
a,
Xiaogang
Liu
a,
Jinyan
Zhao
b,
Hanyu
Wang
a,
Zheng
Liu
c,
Xiaofeng
Yang
 a,
Meishan
Pei
a and
Guangyou
Zhang
a,
Meishan
Pei
a and
Guangyou
Zhang
 *a
*a
aSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: chm_zhanggy@ujn.edu.cn
bJinan Technician College, China. E-mail: zhao_jin_yan@126.com
cKey Laboratory of Superlight Materials and Surface Technology, Ministry of Education, College of Material Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, China. E-mail: liuzhengbeyond@163.com; Tel: +18846135380
First published on 7th November 2018
Abstract
A new fluorescent probe X based on 2-pyridin-2-ylethanamine and benzimidazo[2,1-a]benz[de]isoquinoline-7-one-12-carboxylic acid was designed and synthesized for the detection of Fe3+ and PPi. X showed high sensitivity and selectivity for Fe3+ in the presence of other competing metal ions in DMF/H2O buffer solution. The limit of detection for Fe3+ was calculated from the titration curve to be 6.7 × 10−7 M. The Job's plot confirmed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between the probe and Fe3+, and the complexation stability constant was calculated to be 7.46 × 103 M−1. Moreover, the fluorescence intensity of the probe was recovered after addition of the PPi. X could act as a fluorescence “ON–OFF–ON” probe for the sequential detection of Fe3+ and PPi. The binding mode and sensing mechanism of X with Fe3+ was verified by DFT/TDDFT calculations using Gaussian 09.
1 stoichiometry between the probe and Fe3+, and the complexation stability constant was calculated to be 7.46 × 103 M−1. Moreover, the fluorescence intensity of the probe was recovered after addition of the PPi. X could act as a fluorescence “ON–OFF–ON” probe for the sequential detection of Fe3+ and PPi. The binding mode and sensing mechanism of X with Fe3+ was verified by DFT/TDDFT calculations using Gaussian 09.
1. Introduction
At the present, heavy metal ions are of great concern because they are ubiquitous. Among the various heavy metals, iron, which usually exists as an oxide, is the second most abundant element in the earth's crust, and accounts for about 5% of the elemental content.1–4 Moreover, iron plays an important role in the human body since it is an essential component of hemoglobin, myoglobin and various enzymes; it also participates in many physiological processes at the cellular level, such as oxygen metabolism and DNA and RNA synthesis.5–7 However, both excess or deficiency from the normal permissible consumption limit of iron could lead to physiological disorders and health problems.8,9 An imbalance of iron could cause cell damage and death. Concentration of iron in the human body exceeding a certain limit may cause diseases, such as methemoglobinemia, iron deficiency anemia (IDA), and Parkinson's disease.10–12 Therefore, significant efforts should be made to develop selective and sensitive sensors of Fe3+ that could distinguish Fe3+ from other metal ions in environmental and biological systems.13–15Anions are responsible for a diverse range of applications that are of biological and environmental importance.16 Pyrophosphate (P2O74−, PPi) is a biologically notable anion in many cellular processes, such as DNA and RNA polymerization and enzymatic reactions. Most importantly, the detection of PPi has also been considered to be vital in cancer research.17,18 Considering its essential physiological role, facile monitoring of the concentrations of PPi becomes very crucial. Hence, the design of a probe for detection of PPi is urgent.
Given this discussion, research on carbon-based materials has continued to advance.19–21 The method of detecting Fe3+ by fluorescence sensing has been greatly developed and has gradually replaced the traditional method due to its simple operation, quick response, high sensitivity and selectivity.22–26 At present, numerous excellent fluorescent sensors have been designed and synthesized to recognize iron, such as derivatives based on coumarin, rhodamine and naphthalimide.27–33 1,8-Naphthalimide is a widely used functional material due to its bright color, strong fluorescence, thermal stability, light resistance and rapid response. Therefore, the study of 1,8-naphthalimide derivatives should be paid extensive attention. Recently, a series of fluorescent sensors based on benzimidazo[2,1-a]benz[de] isoquinoline-7-one-12-carboxylic acid derived from 1,8-naphthalimide were designed and synthesized by our group to selectively detect various metal ions.34–41 Benzimidazo[2,1-a]benz[de]isoquinoline-7-one, which contains five conjugated rings, was used as a developmental fluorophore. This heterocyclic compound comprising both benzimidazo and naphthalimide group exhibits both strong extent of conjugation and the biological activity of naphthalimide. These excellent properties also give benzimidazo[2,1-a]benz[de]isoquinoline-7-one a broad potential application as a fluorophore in the field of chemsensors.34,36,38
Furthermore, much attention has been paid on the introduction of various receptors, such as 1-pyridin-2-ylmethanamine and di-2-picolylamine (DPA), to fluorophores for the construction of effective fluorescent probes.42–46 Few studies have been conducted on 2-pyridin-2-ylethanamine. As a recognition group, 2-pyridin-2-ylethanamine could provide N atoms (amino nitrogen and pyridine nitrogen), which are excellent coordinating sites. In this report, sensor X was designed and synthesized based on benzimidazo[2,1-a]benz[de]isoquinoline-7-one as a fluorophore and 2-pyridin-2-ylethanamine as a receptor. As anticipated, the fluorescent probe X could sequentially detect Fe3+ and PPi in DMF/H2O buffer solution.
2. Experimental section
2.1. Materials and methods
All reagents and solvents were used as received from commercial suppliers without further purification. All metal salts (NaCl, LiCl, MgCl2·6H2O, CaCl2, ZnCl2, CdCl2·2.5H2O, HgCl2, AgNO3, CuCl2·2H2O, CoCl2·6H2O, NiCl2·6H2O, MnCl2·4H2O, CrCl3·6H2O, AlCl3·6H2O, FeCl3 and FeSO4) and all anionic sodium salts (Na4P2O7, NaNO3, Na3PO4, Na2S, NaHCO3, NaF, NaS2O3, NaCl, NaBF3, NaHSO3, NaH2PO4, NaBr, Na2CrO4, NaNO2, Na2SO4 and EDTA) were of analytical grade and used without further purification. Stock solutions of the ions mentioned above were prepared with distilled water.2.2. Measurements
UV-vis spectra were obtained on a Shimadzu 3100 spectrometer. Fluorescence spectral data was recorded on an Edinburgh Instruments Ltd-FLS920 Fluorescence Spectrophotometer. Fluorescence measurements were recorded using excitation at 340 nm. The slits of excitation and emission were 10 nm and 10 nm, respectively. 1H NMR measurement was performed on a Bruker AV III 400 MHz NMR spectrometer. 13C NMR spectra data was taken on a Bruker AV III 100 MHz NMR spectrometer with tatramethysilane (TMS) as internal standard and CDCl3 as solvent. Infrared spectral data was obtained on a Bruker Vertex 70 FT-IR spectrometer using samples as KBr pellets. Thin layer chromatography (TLC) analyses were performed to monitor all the reactions.2.3. Sample preparation
All stock solutions of metal salts (NaCl, LiCl, MgCl2·6H2O, CaCl2, ZnCl2, CdCl2·2.5H2O, HgCl2, AgNO3, CuCl2·2H2O, CoCl2·6H2O, NiCl2·6H2O, MnCl2·4H2O, CrCl3·6H2O, AlCl3·6H2O, FeCl3 and FeSO4) and all anionic sodium salts (Na4P2O7, NaNO3, Na3PO4, Na2S, NaHCO3, NaF, NaS2O3, NaCl, NaBF3, NaHSO3, NaH2PO4, NaBr, Na2CrO4, NaNO2, Na2SO4 and EDTA) were prepared at a concentration of 0.03 M. The probe X was dissolved in DMF/H2O (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) buffer solution (10 mM tris, pH = 7.4) at room temperature with the concentration of 1 × 10−5 M.
1) buffer solution (10 mM tris, pH = 7.4) at room temperature with the concentration of 1 × 10−5 M.
2.4. Calculation of quantum yield
Quantum yield was calculated according to the following formula (1):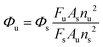 | (1) |
2.5. Calculation of the association constant
The association constant between X and Fe3+ was calculated by the Benesi–Hildebrand eqn (2):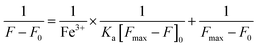 | (2) |
2.6. Theoretical calculations
Density functional theory (DFT) structural optimizations were performed with the Gaussian 09 program. In all cases, the structures were optimized using the B3LYP functional and the mixed basis set 6-31+G (d). Each structure was subsequently subjected to TD-DFT calculation using the B3LYP functional.47 For all optimized structures, frequency calculations were performed to confirm the absence of imaginary frequencies. The molecular orbitals were visualized and plotted with the GaussView 5.0 program.2.7. Synthesis of X
Compound 1 (benzimidazo[2,1-a]benz[de]isoquinoline-7-one-12-carboxylic acid) and compound 2 (benzimidazo[2,1a]benz[de]isoquinoline-7-one-12-carbonyl chloride) were prepared according to a previous report.38![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1662 (C
O), 1662 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1361 (C–N). 1H NMR (400 MHz, CDCl3) δ 9.84 (t, J = 5.4 Hz, 1H), 8.74 (dd, J = 7.3, 1.0 Hz, 1H), 8.66 (dd, J = 4.9, 0.8 Hz, 1H), 8.59 (dd, J = 8.0, 1.1 Hz, 1H), 8.52 (dd, J = 7.3, 0.8 Hz, 1H), 8.30 (dd, J = 7.7, 1.1 Hz, 1H), 8.25 (dd, J = 8.1, 0.6 Hz, 1H), 8.13 (d, J = 7.7 Hz, 1H), 7.82–7.73 (m, 2H), 7.62 (td, J = 7.7, 1.8 Hz, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.32 (d, J = 7.8 Hz, 1H), 7.21–7.14 (m, 1H), 4.10 (dd, J = 12.7, 6.8 Hz, 2H), 3.29 (t, J = 6.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ = 164.91, 160.47, 159.71, 149.37, 149.18, 141.04, 136.59, 135.63, 132.61, 132.14, 131.82, 127.90, 127.21, 127.10, 127.03, 127.01, 125.27, 123.64, 123.39, 122.76, 121.43, 119.63, 118.78, 39.22, 38.21.
O), 1361 (C–N). 1H NMR (400 MHz, CDCl3) δ 9.84 (t, J = 5.4 Hz, 1H), 8.74 (dd, J = 7.3, 1.0 Hz, 1H), 8.66 (dd, J = 4.9, 0.8 Hz, 1H), 8.59 (dd, J = 8.0, 1.1 Hz, 1H), 8.52 (dd, J = 7.3, 0.8 Hz, 1H), 8.30 (dd, J = 7.7, 1.1 Hz, 1H), 8.25 (dd, J = 8.1, 0.6 Hz, 1H), 8.13 (d, J = 7.7 Hz, 1H), 7.82–7.73 (m, 2H), 7.62 (td, J = 7.7, 1.8 Hz, 1H), 7.52 (t, J = 7.9 Hz, 1H), 7.32 (d, J = 7.8 Hz, 1H), 7.21–7.14 (m, 1H), 4.10 (dd, J = 12.7, 6.8 Hz, 2H), 3.29 (t, J = 6.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ = 164.91, 160.47, 159.71, 149.37, 149.18, 141.04, 136.59, 135.63, 132.61, 132.14, 131.82, 127.90, 127.21, 127.10, 127.03, 127.01, 125.27, 123.64, 123.39, 122.76, 121.43, 119.63, 118.78, 39.22, 38.21.
3. Results and discussion
3.1. The synthesis of X
X was synthesized in medium yield according to the synthetic route, as shown in Scheme 1. Compounds 1 (benzimidazo[2,1-a]benz[de]isoquinoline-7-one-12-carboxylic acid) and 2 (benzimidazo[2,1-a]benz[de]isoquinoline-7-one-12-carbonyl chloride) were prepared according to the previous report.38 The structure of X was confirmed by 1H NMR (Fig. S1, ESI†), 13C NMR (Fig. S2, ESI†), FTIR (Fig. S3, ESI†), ESI-MS (Fig. S4, ESI†). All of the data in the spectra were in good accordance with the published structures.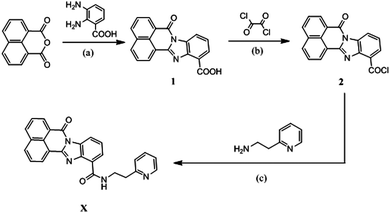 | ||
| Scheme 1 Synthesis routes of X. Conditions: (a) CH3COOH, refluxed, 20 h; (b) CH2Cl2, DMF, r.t., overnight; (c) CHCl3, Et3N, r.t., 1 h. | ||
3.2. The fluorescence properties of probe X towards various metal ions
Primarily, the fluorescence response of X towards various metal ions was evaluated in DMF/H2O (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
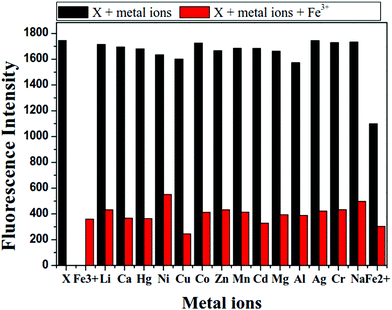
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) buffer solution (0.01 M tris, pH = 7.4). As shown in Fig. 1, X showed suitable fluorescence intensity centered at 498 nm under excitation wavelength of 340 nm. The addition of Fe3+ led to a remarkable fluorescence quenching of X, while negligible response of X was observed towards other common metal ions, including Na+, Li+, Mg2+, Ca2+, Zn2+, Cd2+, Hg2+, Ag+, Cu2+, Co2+, Ni2+, Mn2+, Cr3+, Al3+ and Fe2+. When 20 equivalents of Fe3+ were added to the solution of X, the change in emission color could be clearly observed from yellow-green to colorless under UV light. Correspondingly, the quantum yield (Φ) changed from 0.34 of X to 0.1 of X–Fe complex. Therefore, the probe X exhibited good selectivity towards Fe3+ over other metal ions. This “ON–OFF” switching mechanism could be ascribed to the paramagnetic quenching effect of Fe3+ and/or ligand-to-metal charge transfer (LMCT).48
1) buffer solution (0.01 M tris, pH = 7.4). As shown in Fig. 1, X showed suitable fluorescence intensity centered at 498 nm under excitation wavelength of 340 nm. The addition of Fe3+ led to a remarkable fluorescence quenching of X, while negligible response of X was observed towards other common metal ions, including Na+, Li+, Mg2+, Ca2+, Zn2+, Cd2+, Hg2+, Ag+, Cu2+, Co2+, Ni2+, Mn2+, Cr3+, Al3+ and Fe2+. When 20 equivalents of Fe3+ were added to the solution of X, the change in emission color could be clearly observed from yellow-green to colorless under UV light. Correspondingly, the quantum yield (Φ) changed from 0.34 of X to 0.1 of X–Fe complex. Therefore, the probe X exhibited good selectivity towards Fe3+ over other metal ions. This “ON–OFF” switching mechanism could be ascribed to the paramagnetic quenching effect of Fe3+ and/or ligand-to-metal charge transfer (LMCT).48
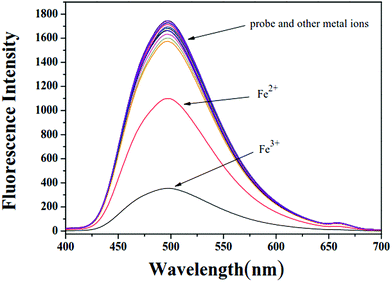 | ||
| Fig. 1 Fluorescence spectra of probe X in DMF/H2O buffer solution upon the addition of various metal ions. | ||
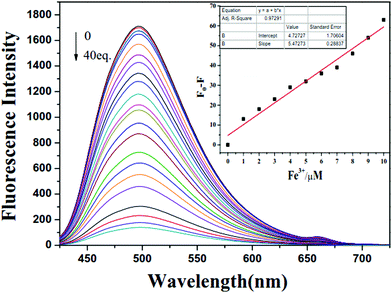
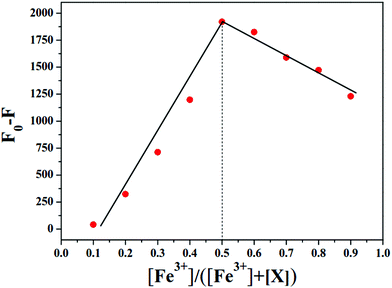
In order to further explore the response properties of probe X to Fe3+, the fluorescence titration experiment in tris buffer solution was performed with gradual addition of Fe3+ to X. The concentration of X was maintained at 1 × 10−5 M, while the concentration of Fe3+ was over the range from 0 to 40 × 10−5 M. As shown in Fig. 2, the free probe X displayed maximum fluorescence intensity at 498 nm. Upon incremental addition of Fe3+ to X, the maximum fluorescence intensity at 498 nm decreased gradually. The fluorescence intensity decreased to a plateau when the concentration of Fe3+ was increased to 40 × 10−5 M (Fig. S5, ESI†). As shown in the inset of Fig. 2, a good linear relationship (R2 = 0.9729) was observed between F0–F and Fe3+ in the range from 1.0 × 10−6 M to 1.0 × 10−5 M. The detection limit was calculated to be 6.7 × 10−7 M based on the formula LOD = 3σ/s, where σ is the standard deviation of blank measurements of 10 times, s is the slope of the linear relationship between F0–F and concentration of Fe3+, where F and F0 were the emission intensities with and without Fe3+, respectively. These results indicated the high sensitivity of X to Fe3+. Moreover, the Job's plot showed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for the binding between X and Fe3+ (Fig. 3). The association constant of X with Fe3+ in DMF/H2O solution was accordingly calculated to be 7.46 × 103 M−1 based on the Benesi–Hildebrand eqn (2) (Fig. S6, ESI†).
1 stoichiometry for the binding between X and Fe3+ (Fig. 3). The association constant of X with Fe3+ in DMF/H2O solution was accordingly calculated to be 7.46 × 103 M−1 based on the Benesi–Hildebrand eqn (2) (Fig. S6, ESI†).
3.3. The UV-Vis spectral studies
The sensing behavior of probe X with different metal ions (Na+, Li+, Mg2+, Ca2+, Zn2+, Cd2+, Hg2+, Ag+, Cu2+, Co2+, Ni2+, Mn2+, Cr3+, Al3+, Fe3+ and Fe2+) was investigated by UV-vis spectroscopy. As shown in Fig. S7 (ESI†), the absorption spectra of X exhibited two bands at 311 nm and 392 nm in DMF/H2O solution. Upon adding 20 equivalents of Fe3+, the absorption intensity was significantly enhanced; however, the addition of other common metal ions did not induce significant changes in the absorption spectrum of probe X, except on addition of Cu2+. As shown in Fig. S8 (ESI†), the absorption titration experiment of X against Fe3+ was performed in DMF/H2O buffer solution. Upon increasing concentration of Fe3+ from 0 to 2 × 10−4 M, the increase in absorption intensity of probe X (1 × 10−5 M) at 315 nm and 365 nm was observed. The absorption spectra changes of X indicated the binding mode between X and Fe3+. There were no evident isosbestic points, which indicated that only the 2-pyridin-2-ylethanamine moiety was involved in the binding. When excited at 392 nm in DMF–H2O (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pH = 7.4) solution, X showed a broad emission brand with the maximum emission at wavelength of 498 nm (Fig. S9, ESI†).
1, pH = 7.4) solution, X showed a broad emission brand with the maximum emission at wavelength of 498 nm (Fig. S9, ESI†).
3.4. Competition experiments with other metal ions
To further prove that the selectivity of probe X to Fe3+ ion was not affected by other metal ions, competition experiments were performed in DMF/H2O buffer solution. As shown in Fig. 4, the probe X (1 × 10−5 M) was treated with 20 equiv. of Fe3+ in the presence of the same concentration of other common metal ions, including Na+, Li+, Mg2+, Ca2+, Zn2+, Cd2+, Hg2+, Ag+, Cu2+, Co2+, Ni2+, Mn2+, Cr3+, Al3+ and Fe2+. This result indicated that the detection of X towards Fe3+ was not interrupted by other metal ions. In Fig. S10 (ESI†), there was almost no decrease in fluorescence intensity when X was treated with 20 equivalent of other common metal ions (Na+, Li+, Mg2+, Ca2+, Zn2+, Cd2+, Hg2+, Ag+, Cu2+, Co2+, Ni2+, Mn2+, Cr3+, Al3+ and Fe2+). However, significant fluorescence quenching was observed with no shift in maximum emission when 5 equiv., 10 equiv., 15 equiv. and 20 equiv. of Fe3+ were respectively added to the above solution. It was found that fluorescence quenching caused by the mixture of Fe3+ with other metal ions was similar to that caused by Fe3+ only. Therefore, the common competing ions did not significantly influence the detection of Fe3+, which confirms the selectivity of X for Fe3+ in DMF/H2O solution.3.5. Effect of pH
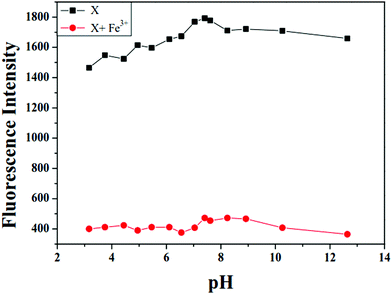
The effects of pH value on the fluorescence spectra of X in the absence and presence of Fe3+ was evaluated. As shown in Fig. 5, the fluorescence intensity of X increased slightly with the change of pH values from 3 to 7.4, and maintained relative stability in the pH range of 7.4–13 due to the protonation and deprotonation of nitrogen atom (pyridine) of X. In addition, on adding 20 equivalent Fe3+ to the buffer solution of the probe X at different pH values, the fluorescence intensity of X–Fe3+ complex was relatively stable at a pH range from 3 to 13. This result indicated that X could detect Fe3+ in a physiological pH range.3.6. Reversible test of probe X towards Fe3+ by PPi
The reversibility and cycling ability properties of X were investigated by testing the probe in presence of some anions. The fluorescence signal response of the X–Fe3+ complex towards various anions were evaluated in DMF/H2O (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
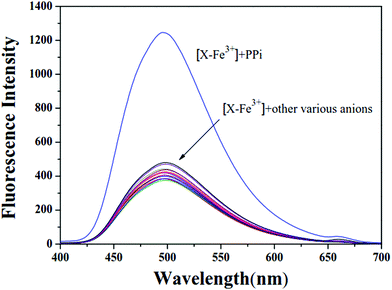
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) buffer solution (0.01 M tris, pH = 7.4). As shown in Fig. 6, X–Fe3+ showed quenched fluorescence (Φ = 0.1). The fluorescence of X–Fe3+ drastically enhanced with the addition of 2 × 10−4 M PPi. In contrast, the addition of other anions (NO3−, PO43−, S2−, HCO3−, F−, S2O3−, Cl−, BF3−, HSO3−, H2PO4−, Br−, CrO42−, NO2−, SO42− and EDTA) to the X–Fe3+ solution did not induce significant fluorescence changes. To validate the practicability of X–Fe3+ as a new fluorescent probe for the detection of PPi in a complicated environment, competition experiments were performed in DMF/H2O solutions. As shown in Fig. S11 (ESI†), the complex X–Fe3+ was treated with 20 equivalents of PPi in the presence of the same concentration of other anions, namely, NO3−, PO43−, S2−, HCO3−, F−, S2O3−, Cl−, BF3−, HSO3−, H2PO4−, Br−, CrO42−, NO2−, SO42− and EDTA. All the chosen competitive anions showed no influence on the fluorescence detection of PPi, suggesting that X–Fe3+ complex had high selectivity for PPi even in the presence of competing anions.
1) buffer solution (0.01 M tris, pH = 7.4). As shown in Fig. 6, X–Fe3+ showed quenched fluorescence (Φ = 0.1). The fluorescence of X–Fe3+ drastically enhanced with the addition of 2 × 10−4 M PPi. In contrast, the addition of other anions (NO3−, PO43−, S2−, HCO3−, F−, S2O3−, Cl−, BF3−, HSO3−, H2PO4−, Br−, CrO42−, NO2−, SO42− and EDTA) to the X–Fe3+ solution did not induce significant fluorescence changes. To validate the practicability of X–Fe3+ as a new fluorescent probe for the detection of PPi in a complicated environment, competition experiments were performed in DMF/H2O solutions. As shown in Fig. S11 (ESI†), the complex X–Fe3+ was treated with 20 equivalents of PPi in the presence of the same concentration of other anions, namely, NO3−, PO43−, S2−, HCO3−, F−, S2O3−, Cl−, BF3−, HSO3−, H2PO4−, Br−, CrO42−, NO2−, SO42− and EDTA. All the chosen competitive anions showed no influence on the fluorescence detection of PPi, suggesting that X–Fe3+ complex had high selectivity for PPi even in the presence of competing anions.
The titration experiments of X–Fe3+ toward PPi were also performed in buffer solutions. As shown in Fig. S12 (ESI†), the fluorescence intensity of X–Fe3+ complex increased gradually with the increase in the concentration of PPi, and then reached the maximum values at about 40 equivalents of PPi without a shift in wavelength. As shown in Fig. S13 (ESI†), a good linear relationship (R2 = 0.9942) was observed between F and PPi in the range from 1.0 × 10−5 M to 1.0 × 10−4 M. The detection limit was calculated to be 2.19 × 10−6 M based on the formula LOD = 3σ/s, demonstrating that X–Fe3+ was a highly sensitive probe for PPi detection in buffer solution.
The reversibility experiments of probe X are shown in Fig. 7. The addition of sodium pyrophosphate (PPi) to the solution containing X and Fe3+ (20 equivalents) immediately restored the fluorescence intensity to the original level of X. This “ON–OFF–ON” fluorescence changes were observed by the alternative additions of Fe3+ and PPi to the solution of X. Furthermore, the fluorescence changes were nearly reversible even after four cycles with the sequentially alternative addition of Fe3+ and PPi. These results indicated that the X–Fe3+ complex with high selectivity and sensitivity could be used as a new sensor to detect PPi in DMF/H2O solution.
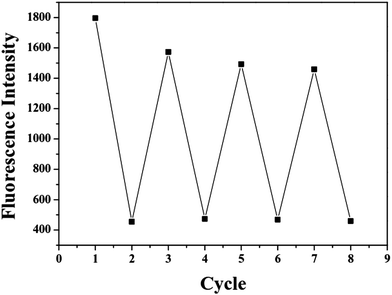 | ||
| Fig. 7 Reversible switching cycles of fluorescence intensity by addition of Fe3+ and PPi (40 equiv., 50 equiv., and 60 equiv.). | ||
3.7. Theoretical calculations
Based on the UV-Vis and fluorescent spectral data (Fig. 3 and Fig. S7, ESI†), evidence for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry ratio of X to Fe3+ was proved. The plausible binding modes for Fe3+ are shown in Scheme 2. Only the nitrogen atoms (amino nitrogen and pyridine nitrogen) in 2-pyridin-2-ylethanamine moiety were involved in the binding with Fe3+. The medium fluorescence of X was likely due to the free 2-pyridin-2-ylethanamine group, which did not have a lone pair of suitable energy for causing a sufficient PET effect in the molecule.37 Fe3+ could strongly quench the fluorescence of the probes because it is paramagnetic with an unfilled d shell.48–52 The optimized structures of probe X and X–Fe3+ complex are shown in Fig. S14 (ESI†), and the optimized structure of the ground state displayed that X provided a highly conjugated π plane with 6 rings containing the fluorophore and pyridine moiety of the receptor.40 Structural optimization and energy calculations provided the best binding mode between X and Fe3+.
1 stoichiometry ratio of X to Fe3+ was proved. The plausible binding modes for Fe3+ are shown in Scheme 2. Only the nitrogen atoms (amino nitrogen and pyridine nitrogen) in 2-pyridin-2-ylethanamine moiety were involved in the binding with Fe3+. The medium fluorescence of X was likely due to the free 2-pyridin-2-ylethanamine group, which did not have a lone pair of suitable energy for causing a sufficient PET effect in the molecule.37 Fe3+ could strongly quench the fluorescence of the probes because it is paramagnetic with an unfilled d shell.48–52 The optimized structures of probe X and X–Fe3+ complex are shown in Fig. S14 (ESI†), and the optimized structure of the ground state displayed that X provided a highly conjugated π plane with 6 rings containing the fluorophore and pyridine moiety of the receptor.40 Structural optimization and energy calculations provided the best binding mode between X and Fe3+.
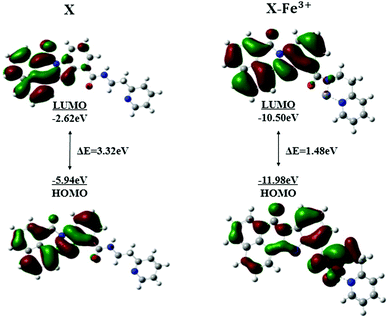
To investigate the coordination of X with Fe3+, energy-optimized structures of X and its corresponding metal complexes were investigated using ab initio density functional theory (DFT) combined with time-dependent density functional theory (TDDFT) calculations, as implemented in the Gaussian 09 package based on B3LYP/6-31G(d) basis. The spatial distributions and orbital energies of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of X and X–Fe3+ complexes were also calculated.42,44 As shown in Fig. 8, in the molecular orbital structure of X (left), the orbitals were localized on fluorophore for both the HOMO and the LUMO, and there was no electron transfer in the excited; hence, X showed yellow-green fluorescence.34 However, the molecular orbital structure of X–Fe3+ complex is clearly different from that of X. The HOMO is mostly located around the metal ions and nearby groups, while electrons in LUMO are localized on the fluorophore. The energy gaps between the HOMO and LUMO in the X and X–Fe3+ were calculated to be 3.32 eV and 1.48 eV, respectively. The energy gaps between the HOMO and LUMO of X–Fe3+ is much lower than that of X. Thus, these results showed that X prefers to coordinate with Fe3+ ions.
4. Conclusions
In summary, a new fluorescent probe X was designed and synthesized based on 2-pyridin-2-ylethanamine as the receptor and benzimidazo[2,1-a]benz[de]isoquinoline-7-one-12-carboxylic acid as a fluorophore. The probe was characterized by NMR and ESI-MS spectroscopy. X displayed high selectivity and sensitivity for Fe3+ over other metal ions in DMF/H2O buffer solution and the detection limit for Fe3+ ion was 6.7 × 10−7 M. The fluorescence intensity of X was recovered after the addition of the PPi. This “ON–OFF–ON” switching process could be repeated several times, and detection of Fe3+ and PPi was not affected in the presence of other competing ions. In addition, the Job's plot showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between X and Fe3+. The binding mode and sensing mechanism of X with Fe3+ was verified by DFT/TDDFT calculation using Gaussian 09.
1 stoichiometry between X and Fe3+. The binding mode and sensing mechanism of X with Fe3+ was verified by DFT/TDDFT calculation using Gaussian 09.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Henan Sanmenxia Aoke Chemical Industry Co., Ltd. w0920 for financial support and Chunxiao Cui for the ESI-MS spectra measurement. Financial support by the National Natural Science Foundation of China (Grants 21708013), the China Postdoctoral Science Foundation (Grants 2017M620288).Notes and references
- N. Singh, N. Kaur, J. Dunn, M. MacKay and J. F. Callan, Tetrahedron Lett., 2009, 50, 953–956 CrossRef CAS.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS.
- H. Zhang, W. Fu, S. Chi and J. Wang, J. Lumin., 2009, 129, 589–594 CrossRef CAS.
- U. Fegade, A. Singh, G. K. Chaitanya, N. Singh, S. Attarde and A. Kuwar, Spectrochim. Acta, Part A, 2014, 121, 569–574 CrossRef CAS.
- M. H. Lee, T. V. Giap, S. H. Kim, Y. H. Lee, C. Kang and J. S. Kim, Chem. Commun., 2010, 46, 1407–1409 RSC.
- Z. Li, L. Zhang, X. Li, Y. Guo, Z. Ni, J. Chen, L. Wei and M. Yu, Dyes Pigm., 2012, 94, 60–65 CrossRef CAS.
- T. Walczyk and F. Blanckenburg, Science, 2002, 295, 2065–2066 CrossRef CAS PubMed.
- G. J. Anderson and F. Wang, Clin. Exp. Pharmacol. Physiol., 2012, 39, 719–724 CrossRef CAS PubMed.
- T. Abe, T. Kinda, Y. Takano, S. Chikazawa, M. Higuchi, N. Kawasaki, K. Orino and K. Watanabe, Biometals, 2006, 19, 651–657 CrossRef CAS.
- M. Y. She, Z. Yang, B. Yin, J. Zhang, J. Gu, W. T. Yin, J. L. Li, G. F. Zhao and Z. Shi, Dyes Pigm., 2012, 92, 1337–1343 CrossRef CAS.
- B. Zhao, T. Liu, Y. Fang, L. Y. Wang, B. Song and Q. G. Deng, Tetrahedron Lett., 2016, 57, 4417–4423 CrossRef CAS.
- N. Narayanaswamy and T. Govindaraju, Sens. Actuators, B, 2012, 161, 304–310 CrossRef CAS.
- J. Huang, Y. Xu and X. Qian, Trans., 2014, 43, 5983–5989 CAS.
- L. Zhang, J. Wang, J. Fan, K. Guo and X. Peng, Bioorg. Med, Chem. Lett., 2011, 21, 5413–5416 CrossRef CAS.
- S. Lia, D. Zhang, X. Xie, S. Ma, Y. Liu, Z. Xu, Y. Gao and Y. Ye, Sens. Actuators, B, 2016, 224, 661–667 CrossRef.
- Z. Li, H. Li, C. Shi, M. Yu, L. Wei and Z. Ni, Spectrochim. Acta, Part A, 2016, 159, 249–253 CrossRef CAS PubMed.
- Z. Li, H. Li, C. Shi, W. Zhang, W. Zhou, L. Wei and M. Yu, Sens. Actuators, B, 2016, 226, 127–134 CrossRef CAS.
- W. Wang, J. Wei, H. Liu, Q. Liu and Y. Gao, Tetrahedron Lett., 2017, 58, 1025–1029 CrossRef CAS.
- Y. Zhang and S. Park, Appl. Catal., B, 2019, 240, 92–101 CrossRef CAS.
- Y. Zhang and S. Park, J. Catal., 2018, 361, 238–247 CrossRef CAS.
- Y. Zhang and S. Park, J. Catal., 2017, 355, 1–10 CrossRef CAS.
- J. N. Yao, W. Dou, W. W. Qin and W. S. Liu, Inorg. Chem. Commun., 2009, 12, 116–118 CrossRef CAS.
- O. Oter, K. Ertekin, R. Kılıncarslan, M. Ulusoy and B. Cetinkay, Dyes Pigm., 2007, 74, 730–735 CrossRef CAS.
- L. Long, L. Zhou, L. Wang, S. Meng, A. Gong and C. Zhang, Anal. Chim. Acta, 2014, 812, 145–151 CrossRef CAS.
- J. Mao, Q. He and W. Liu, Talanta, 2010, 80, 2093–2098 CrossRef CAS.
- S. Devaraj, Y. Tsui, C. Chiang and Y. Yen, Spectrochim. Acta, Part A, 2012, 96, 594–599 CrossRef CAS.
- D. En, Y. Guo, B. Chen, B. Dong and M. Peng, RSC Adv., 2014, 4, 248–253 RSC.
- J. Qin, Z. Yang, G. Wang and C. Li, Tetrahedron Lett., 2015, 56, 5024–5029 CrossRef CAS.
- G. E. Tumambac, C. M. Rosencrance and C. Wolf, Tetrahedron, 2004, 60, 11293–11297 CrossRef CAS.
- M. Kumar, N. Kumar and V. Bhalla, Dalton Trans., 2013, 42, 981–986 RSC.
- T. Puthiyedath and D. Bahulayan, Sens. Actuators, B, 2018, 272, 110–117 CrossRef CAS.
- Q. Meng, Y. Wang, M. Yang, R. Zhang, R. Wang and Z. Zhang, RSC Adv., 2015, 5, 53189–53197 RSC.
- A. Goel, S. Umar, P. Nag, A. Sharma, L. Kumar, Shamsuzzama, Z. Hossain, J. R. Gayen and A. Nazir, Chem. Commun., 2015, 51, 5001–5004 RSC.
- Z. Liu, W. He, M. Pei and G. Zhang, Chem. Commun., 2015, 51, 14227–14230 RSC.
- P. Li and Y. Wang, New J. Chem., 2017, 41, 4234–4240 RSC.
- Z. Liu, C. Peng, C. Guo, Y. Zhao, X. Yang, M. Pei and G. Zhang, Tetrahedron, 2015, 71, 2736–2742 CrossRef CAS.
- Z. Liu, C. Peng, Z. Lu, X. Yang, M. Pei and G. Zhang, Dyes Pigm., 2015, 123, 85–91 CrossRef CAS.
- Z. Liu, Y. Qi, C. Guo, Y. Zhao, X. Yang, M. Pei and G. Zhang, RSC Adv., 2014, 4, 56863–56869 RSC.
- Z. Liu, C. Peng, Y. Wang, M. Pei and G. Zhang, Org. Biomol. Chem., 2016, 14, 4260–4266 RSC.
- Y. Wang, Z. Liu, J. Sun, X. Liu, M. Pei and G. Zhang, J. Photochem. Photobiol., A, 2017, 332, 515–520 CrossRef CAS.
- Y. Zhao, G. Zhang, Z. Liu, C. Guo, C. Peng, M. Pei and P. Li, J. Photochem. Photobiol., A, 2016, 314, 52–59 CrossRef CAS.
- R. Liu, X. Tang, Y. Wang, J. Han, H. Zhang, C. Li, W. Zhang, L. Ni and H. Li, Tetrahedron, 2017, 73, 5229–5238 CrossRef CAS.
- M. J. Chang and M. H. Lee, Dyes Pigm., 2018, 149, 915–920 CrossRef CAS.
- J. F. Zhang, S. Kim, J. H. Han, S. Lee, T. Pradhan, Q. Y. Cao, S. J. Lee, C. Kang and J. S. Kim, Org. Lett., 2011, 13(19), 5294–5297 CrossRef CAS PubMed.
- Y. Fu, C. Fan, G. Liu, S. Cui and S. Pu, Dyes Pigm., 2016, 126, 121–130 CrossRef CAS.
- M. L. Giuffrida, G. T. Sfrazzetto, C. Satriano, S. Zimbone, G. A. Tomaselli, A. Copani and E. Rizzarelli, Inorg. Chem., 2018, 57, 2365–2368 CrossRef CAS.
- M. J. Frisch, G. M. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb and J. R. Cheeseman, et al., GAUSSIAN 09 (Revision D.02), Gaussian Inc., Pittsburg, PA, 2006 Search PubMed.
- W. Wang, J. Wu, Q. Liu, Y. Gao, H. Liu and B. Zhao, Tetrahedron Lett., 2018, 59, 1860–1865 CrossRef CAS.
- C. Pan, K. Wang, S. Ji, H. Wang, Z. Li, H. He and Y. Huo, RSC Adv., 2017, 7, 36007–36014 RSC.
- T. G. Jo, J. M. Jung, J. Han, M. H. Lim and C. Kim, RSC Adv., 2017, 7, 28723–28732 RSC.
- C. Wang, J. Fu, K. Yao, K. Xue, K. Xu and X. Pang, Spectrochim. Acta, Part A, 2018, 199, 403–411 CrossRef CAS.
- Z. Li, L. Zhang, W. Zhao, X. Li, Y. Guo, M. Yu and J. Liu, Inorg. Chem. Commun., 2011, 14, 1656–1658 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nj04870c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |