Nanoscale mapping of hydrogen evolution on metallic and semiconducting MoS2 nanosheets†
Abstract
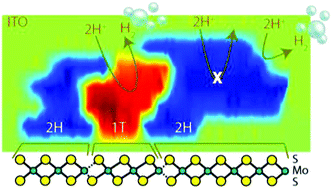
Hydrogen evolution reaction (HER) on molybdenum disulfide (MoS2) nanosheets is enhanced for the metallic (1T) phase relative to the thermodynamically stable semiconducting (2H) phase. To measure this difference, we employ scanning electrochemical microscopy (SECM) for high-resolution mapping (<20 nm spatial resolution) of surface reactivity for mixed-phase and pure 2H-only MoS2 nanosheets. For mixed-phase MoS2 nanosheets, we find major differences in reactivity of the two phases for electron transfer involving ferrocenemethanol, allowing us to locate 1T and 2H regions and directly map the corresponding HER activity. In our measurements, we find that HER is immeasurably slow on the 2H basal plane and much faster on edges, whereas 1T portions are highly reactive across the entire portion. We also use scanning transmission electron microscopy-electron energy loss spectroscopy and scanning Kelvin probe microscopy to corroborate the phase domains and local workfunctions (surface potentials) within the MoS2 nanosheets; the mixed-phase MoS2 has a shallower workfunction compared to 2H MoS2, which could enable a greater driving force for H2 generation. This powerful combination of techniques for spatially mapping surface reactivity and correlated phase domains should be applicable to a broad range of materials for HER and other catalysis reactions.


 Please wait while we load your content...
Please wait while we load your content...