Carbon dots produced via space-confined vacuum heating: maintaining efficient luminescence in both dispersed and aggregated states†
Abstract
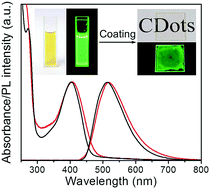
Aggregation-induced quenching (AIQ) of emission is an obstacle for the development of carbon dots (CDots) for solid-state luminescent devices. In this work, we introduce a method to avoid AIQ and to produce highly luminescent CDots through a space-confined vacuum heating synthesis. In the presence of CaCl2, a mixture of citric acid and urea forms an inflated foam under vacuum heating at 120 °C. Upon gradually increasing the heating temperature to 250 °C, blue emissive molecular species are first formed, and are then transformed into uniform-sized green emissive CDots through dehydration and carbonization processes taking place in the confined ultrathin spaces of the foam walls. The green luminescence of these CDots originates from conjugated sp2 domains, and these CDots exhibit a high photoluminescence quantum yield (PLQY) of 72% in ethanol solution. Remarkably, due to the existence of only one type of recombination center in these nanoparticles, AIQ does not take place in CDot-based close-packed films, which show strong emission with a PLQY of 65%. Utilizing the differences in the emission properties of vacuum heating produced CDots, CDots synthesized through microwave-assisted heating, and commercial green fluorescent organic ink (namely, excitation-dependent vs. excitation-independent emission, and different stability against photobleaching), multilevel data encryption has been demonstrated.
- This article is part of the themed collection: International Year of the Periodic Table : Low Dimensional Carbon Systems


 Please wait while we load your content...
Please wait while we load your content...