DOI:
10.1039/C8NH00191J
(Review Article)
Nanoscale Horiz., 2019,
4, 557-578
Advances in controlled gas-releasing nanomaterials for therapeutic applications
Received
19th July 2018
, Accepted 22nd January 2019
First published on 23rd January 2019
Abstract
In the field of nanomedicine, gas therapy is currently a hot topic under exploration as it can treat a variety of disease conditions. The key challenge of delivering medicinal gas via any delivery system is that the technique should be feasible to deliver the gas over an appropriate time interval as well as specifically targeting the affected area. This has enabled gas therapy as an active research area with an aim to improve and discover new materials, methodologies and technologies. In this review, we present the recent advances in research on delivering medicinal gases using nanocarriers that can specifically target with precise spatial-temporal control of release behavior and discuss their future perspectives. The main emphasis has been focused on nanoparticle gas carriers to overcome the challenges in gas delivery for therapeutic applications including prevention of gas diffusion while transportation, improving the stability of the gas in the complex biological environment, specifically targeting the tissue and controlled gas release for efficient programmed treatment modality. Furthermore, the therapeutic effects of the nanomaterial gas carriers via efficient gas releasing properties demonstrated in the preclinical studies with cell/animal models are discussed. This critical review is intended to make clear the present status, the possibility and future advancement of gas therapy for the scientific community.

Divinah Manoharan
| Divinah Manoharan received her PhD degree from Anna University, India, in 2013 and worked as a Post-doc at Tamkang University and National Cheng-Kung University, Taiwan. Her research interest is mainly focussed on the design and synthesis of novel multifunctional nanostructured materials for bio-medical applications. |

Wei-Peng Li
| Wei-Peng Li received his PhD degree in Chemistry from National Cheng Kung University, Taiwan, in 2015. And then, he worked as a postdoctoral fellow at the Department of Chemistry, National Cheng Kung University, Taiwan. His research interests are focused on the development of nanomaterials, and applying them for therapy and diagnosis of cancer. |

Chen-Sheng Yeh
| Chen-Sheng Yeh received his PhD degree in Chemistry from the University of Georgia, USA, in 1993. He then worked as a postdoctoral fellow at the Department of Chemistry, Purdue University, USA. He started as an Associate Professor at the Department of Chemistry, National Cheng Kung University, Taiwan, in 1995. He was a full Professor in 2001, and then was promoted as a Distinguished Professor in 2009 and a Chair Professor in 2017. He was awarded the excellent research award by the Ministry of Science and Technology, Taiwan, in 2010 and 2016. His research focuses on the development of functional nanomaterials for biological applications and the area of nanostructure characteristics. |
1. Introduction
Various gases produced in mammalian cells play a vital role in biological activities and act as signaling molecules (transmitters) in the human body. A well-known gas with regard to the biological system is oxygen which is essential for respiration to sustain life. Apart from that, there are various gases, namely, NO, CO and H2S called the gaseous triumvirate, that act as biological mediators/gaseo-transmitters (Fig. 1). Carbon monoxide (CO) is produced by heme degradation by heme oxygenase and is transported to the cell through the heme proteins’ K/Ca channel receptors. CO located inside the cells serves many functions that include maintaining cellular balance, activating the bio-synthesis, and regulating the expressions of genes and proteins.1 In fact, multi-pathways exist for CO to achieve therapeutic effects. For example, when cancer cells are treated with a high concentration of CO, the anti-Warburg effect leads to cell apoptosis following CO-responsive metabolic exhaustion.2 For cytoprotection, CO could protect the cells by preventing peroxynitrite-induced apoptosis via the inhibition of KV2.1 channels, reducing mitochondrial membrane permeabilization, and regulating cytochrome c release.3 In addition, intracellular CO and CO-induced NO formation in the cells could increase the reactive oxygen species (ROS) generation from the mitochondria to inhibit the L-type Ca2+ current for cytoprotection.4 These facts prove the therapeutic potential of CO in neurological and cardiovascular damage. Based on the conventional methods for CO delivery, CO-releasing molecules (CORMs) are often used to carry CO into the living systems and spontaneously release CO through enzyme catalysis. Nitric oxide (NO) is produced by L-arginine by nitric oxide synthase or reduction of nitrite and enters the cell via heme proteins’ K/Ca channel receptors. NO is a highly active molecule and acts as a key regulator in the body especially in the regulation of cyclic guanosine monophosphate (cGMP).5 It is an important signaling molecule with characteristics to stimulate smooth muscle cells inside the vascular wall to expand the blood vessels for increasing blood flow and decreasing blood pressure. Therefore, NO is seen as a beneficial gas molecule for the prevention of thrombus formation and cardiovascular diseases. In addition, NO can also regulate various signal pathways in the cells to prevent injury from ischemia/reperfusion (I/R).6 NO could decrease the ROS generation through the inhibition of cytochrome oxidase in the mitochondria. NO is able to reduce the expression of TNF-α, which involves the inhibition of NF-κB to down-regulate JNK and ERK expressions, as well as p38 to decrease the activity of caspase-3 and p53. Upon down-regulating the JNK, ERK, and p53, the cell inflammation is reduced. NO increases the cGMP levels, which leads to the inhibition of caspase-3 activity. The lower expressions of caspase-3 and p53 can reduce cell apoptosis. Overall, NO protects the organs by preventing the injury from I/R through NO-induced inhibition of ROS generation, anti-cell inflammation and reduction of cell apoptosis. Hydrogen sulphide (H2S) is produced in the biological system from L-cysteine by cystathionine β synthase and cystathionine γ lyase and is recognized by KATP channels. H2S is a signaling molecule with characteristics of vasodilation and helps prevent cardiovascular diseases. H2S shows a protective effect in heart injury through multiple pathways including the activation of ERK, Akt and KATP channels.7 H2S can also promote NO production and mediate cGMP/PKG signaling for vasodilation.7 Moreover, H2S has the therapeutic potential in chemotherapy-induced cardiotoxicity through various pathways such as inhibition of the p38 MAPK pathway, activation of gp130/STAT3 and PI3K/Akt, etc.8 These gaseotransmitters act as vasodilators as well as anti-inflammatory and cytoprotective mediators when administered in low physiological dose. At the clinical or preclinical stage of progress, therapeutic strategies are constructed depending on the different traits of gaseotransmitter pharmacology. Nevertheless, other gases like carbon dioxide (CO2) also show a therapeutic effect by increasing the oxygen release from hemoglobin (Hb) and improving the blood circulation around the wound sites to accelerate wound healing based on the Bohr effect.9 The CO2 entering the tissue capillaries promotes the release of oxygen by decreasing the blood O2 affinity. The Bohr–Haldane effect explains the cellular respiration and the transport of blood gases, namely, CO2 and O2, by Hb. Interestingly, recognizing local O2 demand through Hb deoxygenation, the red blood cells (RBCs) can regulate the blood flow by the release of vasodilatory compounds equivalent to the degree of deoxygenation.10 RBCs release adenosine triphosphate (ATP) in response to low O2 levels and the extracellular ATP subsequently binds to purinergic receptors causing vasodilation, partly via stimulation of NO synthesis and release from vascular endothelial cells.11,12 On the other hand, CO2 can also induce cell apoptosis via a mitochondrial pathway. CO2 induces the overexpression of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) leading to the proliferation of mitochondria and results in cellular apoptosis.13 All these therapeutic effects make gas therapy a vital treatment modality for prospective medical applications and bring the design of nanocarriers into the spotlight to accomplish efficacious treatment strategies.
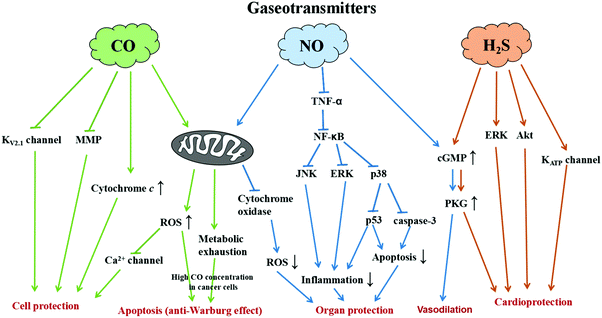 |
| | Fig. 1 Illustration showing the signal pathways of gaseotransmitters for various therapeutic applications. | |
The toxic effects of reactive oxygen species (ROS) destruct cell constituents, i.e. lipids, proteins and DNA, thereby ultimately leading to cell death. Although the ROS act as a molecular signal to activate the immune system for antibacterial defense, excessive levels of ROS result in oxidative stress causing cellular injury.14 The oxidative stress leads to many diseases including cardiovascular disease, cancer, chronic inflammatory disease, hypertension, ischemia/reperfusion injury, acute respiratory distress syndrome (ARDS), neuro-degenerative diseases such as Parkinson's disease and Alzheimer's disease, and to aging. Recently, the use of pharmaceutical gases to treat oxidative stress is evolving as a potential therapeutic possibility. Exogenous administration of antioxidant therapeutic gases evidences the ability to defend against oxidative stress and averts the pathological processes of a wide range of diseases.15 It is noted that a higher dose of these gases is actually lethal whereas, in contrast, a lower dose is considered safe and essentially therapeutic. Most common clinical methods of gas administration are inhaling the gas via lung ventilation, gas exposure on an open wound, drinking bubble water for intake in the digestive tract, and injecting gas containing saline into the vein. Currently, there are diverse gas administration systems in use as well as critically subject to advancement; nevertheless, the crucial concern relies on providing safe gas delivery to patients with precise dosage and stringent process monitoring. Apart from the advancement of safety devices for gas inhalation, prospective clinical applications may include a parenteral injection or drug containing gas-releasing moieties.
The key challenge of delivering gas via any kind of transportation system is that the technique should be feasible to deliver the gas over an appropriate time interval as well as specifically targeting the affected area without any complication to other organs or systems to attain effective therapeutic results. The concerns of using gaseous molecules for medicinal therapy include cost, availability, their environmental activity and reactivity upon accidental exposure, the safety of storage and transportability; all these considerations of handling gaseous molecules render their use particularly impractical. Another strategy is the use of chemical donors which act as gas releasing molecules. For example, CO and NO gases can be carried through using inorganic metal complexes with carbonyl ligands (CORMs) or nitrosyl ligands such as the nitric oxide releasing materials (NORMs), respectively.16,17 The gas can be released when the complexes receive a trigger like UV-light exposure or enzyme/solvent catalysis.18,19 However, CORMs and NORMs have some fundamental disadvantages such as poor accumulation in the target position and less controllable gas release in deep tissue. In addition, the metal complexes have the features of potential bio-toxicity and poor solubility in blood posing a big challenge to CORMs and NORMs applied in clinical medicine. These chemical donors also impose certain critical limitations which need to be primarily considered. It is clear that there is a high demand for more effective and efficient gas therapy capable of delivering a therapeutic level of gas dosage to the diseased organ or system with minimum or no side effects. Accordingly, this has rendered gas therapy an active research area with an aim to improve and discover new methodologies and technologies. For instance, the gas from the chemical donors can be delivered effectively via loading them in nanoparticles (NPs), which is expected to influence the bio-distribution greatly as the chemical donor loaded NPs can be delivered with spatio-temporal accuracy with modified pharmacokinetic properties. In this context, the use of gases as therapeutic tools for a variety of disease conditions is right now a hot topic under exploration in the field of nanomedicine as evidenced from the recent research marking the improvement on medical gas therapies. In this article, we review the recent advances in research on delivering medical gases with therapeutic properties using a nanocarrier that can specifically target with precise spatial-temporal control of release mechanisms and discuss their future perspectives.
2. Design of nanocarriers for generation/release of gas in situ
Gas transportation is a challenge because of the high diffusion rate and low solubility of gases in blood. Consequently, their movement, accumulation, penetration and action on targets are difficult. Therefore, the use of nanocarriers to transport gas is an important approach to improve the efficiency of gas therapy. Hence, well-designed nanocarriers are a promising development for gas delivery through efficient gas transport, generation and release in bio-applications. The gas can be loaded on to nanomaterials through different means. Mesoporous NPs with a high specific surface area are advantageous to load a significant amount of gas molecules.20 Gases can be filled in the core of a polymer shell in the form of a nano-balloon.21 Different from the strategy of gas loading, the other pathway involves gas ligands (NO and CO) directly grafted onto the metal atoms on the surface of NPs. For example, an N-diazeniumdiolate NO donor was conjugated with the polymer chain forming NO self-generated polymer NPs.22 Therefore, it is obvious that the gas can be carried by nanomaterials with specific designs for specific demands. Furthermore, the ability of the targeting function added to the NPs carrying the gas could significantly improve the therapeutic efficiency, reduce the side-effects, and decrease the amount of nanocarriers. For example, the inclusion of an antibody can track the disease related cells following uptake via endocytosis.23,24 NPs can be conjugated with a specific recognition molecule such as aptamers to enhance active targeting.25 For the purpose of targeting tumor cells, the NPs can be modified with a targeting polymer, for example, mercapto group-poly(ethylene glycol)-folic acid.26 Using magnetic NPs as the carrier can allow the transport of gas to the specific location via magnetic guiding.27,28
Control of gas release from the nanocarrier in a suitable time period and location is highly desirable. The development of well-designed nanocarriers is thus a crucial work in order to reach the goal of gas delivery and controllable release. The NPs have been potentially designed to simultaneously enable the function of drug delivery and controlled release by various triggers including hyperthermia,29,30 microwave,31 pH-responsive processes,32 carrier disruption/degradation,33 and photo- or enzyme-induced bond break between the carrier and drug.34 For instance, gold (Au) NPs can generate heat by absorbing a specific wavelength irradiation corresponding to the surface plasma resonance, giving rise to a photothermal effect.29,35,36 The temperature increase can be utilized for drug release according to the designed architecture following the heat-induced dehybridization of double-stranded DNA blockers from mesoporous SiO2 shell-coated Au nanorods.30 Drug-loaded liposomes are well known to serve as a clinical medication to treat diseases based on the degradation feature of liposomes having the behavior of slow drug release.32,33 Drug release from Au NP stabilized liposomes through increasing pH is demonstrated.34 The stable Au NP-modified liposomes were fabricated at pH 1.2, while the rupture of the liposomes was seen at pH 7.4 for drug release. In addition, the intracellular environment in lysosomes revealing an acidic environment (pH ∼ 5) can also be utilized to trigger the drug release from a pH-responsive system following the endocytosis process.33 The use of biodegradable NPs formed via self-assembly is also a yet another option.37 Drug vehicles designed with carbohydrate and protein-based materials will offer bio-compatibility as well as render suitability for diseases with considerably longer treatment.38 Some studies have shown that the drugs can be grafted on the NPs using the labile molecule as a linker to connect drugs with NPs. And then, the linker was cleaved upon UV light exposure or enzyme-induced drug release.34 Moreover, upconversion NPs can be employed as a UV light emitter when exposed to near-infrared (NIR) light to eliminate the doubts about using UV light for medical applications.39 Overall, the various nanomaterials have unique characteristics and functions making them potential candidates as gas nanocarriers for gas therapy applications. The strategies that can be adopted to circumvent the barriers in gas therapy via nanocarriers are discussed in detail in the following sections. Table 1 shows a brief summary of gases, types of nanomaterials, specific simulation and its benefits in this review.
Table 1 Summary of the strategies of gas release using nanomaterials
| Gas(es) |
Type of nanomaterial(s) |
Approach(es) of gas release |
Function(s) |
Ref. |
| NO |
Al-MCM-41 NPs |
Light |
Anti-microbial |
43
|
| NO |
Mesoporous silica NPs |
Spontaneous release |
Anti-microbial |
51
|
| NO |
Dendrimer NPs |
Spontaneous release |
NA |
55
|
| NO |
Micelles |
Spontaneous release |
Cancer therapy |
59
|
| NO |
Vitamin A decorated polymeric NPs |
Spontaneous release |
Treatments of both liver fibrosis and portal hypertension |
64
|
| NO |
PLGA@PEG micelles |
Light |
Cancer therapy |
81
|
| NO |
Mesoporous silica NPs |
Light |
NA |
82
|
| NO |
Graphene oxide nanosheets |
Light |
Cancer therapy |
90
|
| NO |
UCNP@SiO2 NPs |
X-ray |
Cancer therapy |
94
|
| NO |
Au NPs |
Spontaneous release |
Vasodilation |
105
|
| NO |
Cu1.6S NPs-embedded polymersomes |
Light |
Vasodilation |
98
|
| NO |
Poly(lactic-co-glycolic acid)–polyethylenimine NPs |
NA |
Wound healing |
101
|
| NO |
Dendrimeric prodrug@hyaluronicacid shell NPs |
Enzyme |
Cancer therapy |
67
|
| NO |
Hollow mesoporous silica NPs (HMSNs) |
Ultrasound, H2O2 |
Cancer therapy |
70
|
| NO |
Superparamagnetic iron oxide-encapsulated mesoporous silica NPs (SPION@hMSN) |
Ultrasound |
Cancer therapy |
69
|
| NO |
Hollow mesoporous organosilica nanoparticle |
Glucose |
Cancer therapy |
75
|
| NO |
Magnetic microvesicles loaded with glucose oxidase |
Glucose |
Cancer therapy |
76
|
| NO |
UCNP-RBS |
Light |
Cancer therapy |
91–93
|
| CO |
Poly(L-lactide-co-D/L-lactide) fibers |
Light |
NA |
46
|
| CO |
Al-MCM-41 NPs |
Light |
Cancer therapy |
44
|
| CO |
Al-MCM-41 NPs |
Light |
Vasodilation |
45
|
| CO |
Polymeric framboidal NPs |
Cysteine |
Anti-inflammatory |
65
|
| CO |
HMSNs |
H2O2 |
Cancer therapy |
74
|
| CO |
UCNPs |
Light |
NA |
39
|
| CO |
Graphene oxide nanosheets |
Light |
Anti-inflammation |
83
|
| CO |
Prussian blue NPs |
Light |
Cancer therapy |
84
|
| H2S |
Liposomes |
Enzyme |
Cancer therapy |
63
|
| H2S |
Micelles |
Cysteine |
Cancer therapy |
66
|
| CO2 |
CuS NPs |
Light |
Wound healing |
49
|
| O2 |
MnO2 NPs |
H2O2 and H+ |
Reducing hypoxia area in tumors |
61
|
| O2 |
Carbon nitride nanosheets |
Light |
Reducing hypoxia area in tumors |
62
|
| O2 |
Albumin stabilized perfluorocarbon nanodroplets |
Ultrasound |
Reducing hypoxia area in tumors |
71
|
2.1. Prevention of gas diffusion via loading on the carriers
Generally, the fast diffusion rate of gases causes poor interaction of the gas molecules with the targeted bio-system compared to the solid and liquid forms.40 Accordingly, gas administration in clinical medicine is not feasible except for the conventional inhalation method. The applications of gas therapy are greatly limited due to the difficulty in administration of those gases which are unsuitable for use by inhalation. The other gas treating methods like drinking bubble water, injection of gas-containing saline, and body exposure to a gas-filled environment all showed poor efficiency thereby reducing their applicability.41,42 However, nanocarriers can act as a host for gas or gas-generating agents which can overcome the issue of spontaneous diffusion of gases. The strategies of using the nanocarriers to transport gas into the body are promising.
Mesoporous aluminosilicate-based host (Al-MCM-41) loaded with a photoactive NO donor, manganese nitrosyl ([Mn(PaPy3)(NO)](ClO4)) could rapidly release a high dosage of NO upon exposure to 600 nm light to kill both drug-susceptible and drug-resistant Acinetobacter baumannii (Fig. 2).43 Similarly, PhotoCORMs entrapped within the pores of Al-MCM-41 NPs (size ∼ 100 nm) enabled the burst of CO upon irradiation and allowed luminescence tracking of CO release within the target cell without the need for a secondary reported appendage.44 Notably, photoCORMs entrapped within the pores of Al-MCM-41 NPs (size ∼ 60 nm) rapidly released CO upon visible light irradiation which was also shown to induce vasorelaxation in rat aortic muscle rings.45 The photosensitive CO-releasing molecule, dimanganese decacarbonyl (CORM-1), was non-covalently embedded into nanoporous non-woven poly(L-lactide-co-D/L-lactide) (PLA) fibers via electrospinning to enable light triggered CO release.46
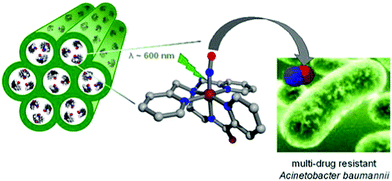 |
| | Fig. 2 Mesoporous aluminosilicate-based host (Al-MCM-41) loaded with a NO donor. Reprinted with permission from Heilman et al.43 Copyright© 2012 American Chemical Society. | |
Another well-known porous material, the iron MOF of the MIL-88 structure type, was applied for NO adsorption and delivery. The iron MOF provided narrow pores and Fe2+/Fe3+ active sites for physisorbed and chemisorbed NO, respectively. A high amount of NO loading was obtained and showed a small amount of NO release in the simulated body fluid indicating high stability during the NO delivery process.47 Biocompatible and stable porous boron nitride was synthesized showing excellent capability for CO2 capture. The porous type of boron nitride powders can be produced by tuning the synthetic conditions to exhibit distinct gas adsorption behaviors.48 Recently, mesoporous CuS NPs were modified with cysteamine–dopamine conjugates via S–Cu bond formation providing hydroxyl groups for iron ion coordination. Meanwhile, bicarbonates were chelated to the exposed iron sites on the NPs. The heat generated from CuS NPs upon NIR light exposure enabled the release of CO2 from bicarbonates, which accelerated wound healing (Fig. 3).49 NO-NPs are hydrogel/glass composites with nanosized building blocks with various degrees of aggregation of the uniform sized NPs (each individual element unit ∼10 nm) composed of a silane derived sol–gel matrix, chitosan, glucose, PEG and sodium nitrite (NO donor). Since the silane derived sol–gel is very porous in structure and not suitable for trapping small solvent and solute molecules including water, nitrite and NO, the authors used a glass forming material like glucose to overcome the high porosity limitation by “plugging” the pores of the sol–gel with a relatively stable (when dry) hydrogen-bonded network of glass forming molecules. The glassy matrices required a thermal reduction process to drive the conversion of nitrite to NO. In their other work, the authors reported that the inclusion of chitosan and PEG as the additives to the basic sol–gel preparation proved to be effective with respect to NO formation, NO retention and slow sustained release of NO. This material could be easily converted to a powder composed of NPs capable of delivering therapeutic amounts of NO upon topical application for overextended time periods, which enhanced wound healing.50 Hetrick et al. demonstrated that NO-releasing silica NPs, prepared via co-condensation of tetraalkoxysilane with aminoalkoxysilane modified with diazeniumdiolate NO donors, allowed the storage of large NO payloads to kill Pseudomonas aeruginosa because of the novel anti-microbial activity without being non-toxic to healthy cells.51
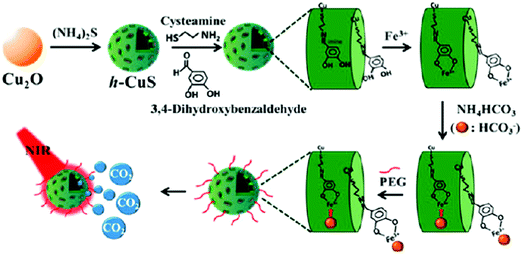 |
| | Fig. 3 Scheme of design of h-CuS NPs for CO2 release upon NIR light exposure. Reprinted with permission from Li et al.49 Copyright© 2017 American Chemical Society. | |
The surface of nanocarriers can also serve as a delivery host to carry gaseous donors. Different from porous structures, graphene oxide is a 2-D material having the potential for H2 absorption after anchoring Ti atoms. The H2 can coordinate with the Ti atoms on the graphene oxide sheet to form hydrogenated Ti-anchored graphene oxides, which are stable without the occurrence of Ti clustering.52 An aluminum nitride (AIN) based material was prepared as the H2 gas carrier displaying H2 molecules bound on the sites of Al atoms.53 Not limited to the 2-D structure, the spherical nanomaterials can readily carry gas donors as well. S-nitrosothiol was modified on the surface of silica spheres for carrying and releasing NO.54 A case of dendrimer-type spherical carrier revealed that the secondary amine dendrimer conjugates of amine-grafted polypropylenimine dendrimers displayed high storage capacity of NO and gas release under physiological conditions (Fig. 4).55
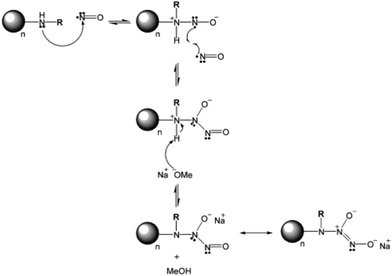 |
| | Fig. 4 Common method for NO modification on the surface of amine group-exposed NPs. Reprinted with permission from Stasko et al.55 Copyright© 2006 American Chemical Society. | |
Soft materials are suitable for encapsulating gas or gaseous donors in the form of micelles and vesicles. Emulsion preparation was employed to make phospholipid based H2-loaded microbubbles that displayed excellent performance with respect to the H2-induced prevention of myocardial ischemia-reperfusion injury as well as the diagnosis via in situ ultrasound imaging.56 Using triblock copolymers consisting of a hydrophilic poly(ethylene glycol) block, a poly(ornithine acrylamide) block connected with CORMs, and a hydrophobic poly(n-butylacrylamide) block, micelles were produced for CO release in immunotherapy.19 A potential oxygen carrier was made from poly-(2-methyloxazoline)–poly(dimethylsiloxane)–poly(2-methyloxazoline), which formed vesicles with the hemoglobin (Hb) embedded inside, serving as an oxygen transport protein.57 Moreover, Hb microspheres can be synthesized by a layer-by-layer assembly process using calcium carbonate (CaCO3) particles as templates. The biocompatibility of the Hb microspheres was obtained after removing CaCO3 for oxygen delivery (Fig. 5).58 PEI based micelles were assembled with poly(L-lactide) and PEG via a click reaction. The hydrophobic poly(L-lactide) and abundant secondary amines from PEI can be utilized to absorb hydrophobic drugs (paclitaxel) and chemosensitizers (NO), respectively. Through NO-enhanced chemo-efficacy, this strategy has the potential for anti-MDR tumor treatment.59
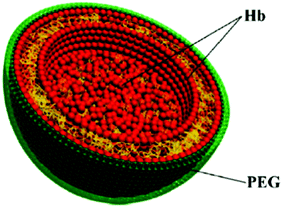 |
| | Fig. 5 Hb-composed microsphere for gas delivery. Reprinted with permission from Duan et al.58 Copyright© 2012 American Chemical Society. | |
2.2. Carrying the gas/gas donor to a specific target
In general, inhaling the gas leads to gas distribution to all places through blood circulation. Because diseases and biological conditions like tumor, thrombus, wound, ischemia-reperfusion injury, and brain-related diseases usually appear in a specific location, conventional gas therapy cannot fully localize the treatment to a specific area. Then, the efficiency of treatment is not optimal because of the lack of sufficient concentration of gas delivered at the required site. In addition, excessive gas is dispersed into the healthy areas, which causes potential risks and possible side-effects for patients. NPs are suitable for specific targeting. For example, NPs can accumulate better at the tumor tissue compared to the microparticles and small molecule drugs through the enhanced permeability and retention (EPR) effect. In addition, the molecular grafting technology is feasible for the surface modification of NPs to attain the goal of targeting.23,60 Various antibodies were demonstrated to assist the NPs to find a specific target through a receptor-mediated process.24 The hypoxia region in a solid tumor is of major concern because it leads to poor efficiency of chemotherapy, radiotherapy and photodynamic therapy. Therefore, an oxygen supply strategy could be a better solution for the resistance issue in tumors. A study of mannan-hyaluronic acid conjugated coated manganese dioxide (MnO2) NPs demonstrated accumulation ability in the hypoxia region with mannan-receptor expression in tumor-associated macrophages (TAMs), which are the macrophages residing in the hypoxia region to assist the cancer cells in proliferation and chemoresistance. The MnO2 NPs inside the TAMs reacted with the hydrogen peroxide (H2O2) in the acidic environment that immediately generated oxygen to overcome the hypoxia-related effect in tumor therapy. Furthermore, the hyaluronic acid was also beneficial for the reprogramming of TAMs to anti-tumor M1 macrophages improving the normoxia process.61
A carbon dot doped carbon nitride (C3N4) based nanocomposite (PCCN) was used for red light-triggered oxygen production by water splitting, which is a reaction for the transformation of H2O into H2 and O2. In this study, the photosensitizer protoporphyrin IX (PpIX) and tumor-targeting sequence RGD (Arg–Gly–Asp) were used for photodynamic therapy (PDT) and cancer cell targeting, respectively. The H2O2-free strategy for oxygen generation in this case without the need for intracellular H2O2 concentration (<50 μM) revealed remarkable results of increasing the oxygen level in tumors (Fig. 6).62 An interesting design shows liposomes encapsulating magnetic nanoparticles in the core and the hydrophobic anethole dithiolethione (ADT) as an H2S donor in the phospholipid shell. The magnetic liposomes can be accumulated in the tumor site through both the EPR effect and magnetic targeting operation. H2S microbubbles were generated from ADT via an enzymatic reaction after liposome rupture that was remotely controlled through external magnetic field exposure. The large amount of H2S microbubbles caused the destruction (HepG2) and growth inhibition of cancer cells by the cavitation effect and anticancer effect, respectively, as well as provided real-time ultrasound imaging for diagnosis (Fig. 7).63 Efficacious targeting was achieved by using vitamin A decorated polymeric NPs loaded with GSNO (VA-GSNO-NPs) for intracellular NO release. The administration of VA-GSNO-NPs apparently decreased the portal pressure in bile duct-ligated rats by about 25% (∼12 mmHg), while having minimal influence on arterial pressure evidencing the specific delivery of NO into the stellate cells in the liver using targeted NPs. Thus, the HSC targeted delivery of NO is obviously a significant promise to the health issue of widespread importance where there are few therapeutic options.64 These above-discussed cases prove the boundless opportunity for gas treatment via specific targeting.
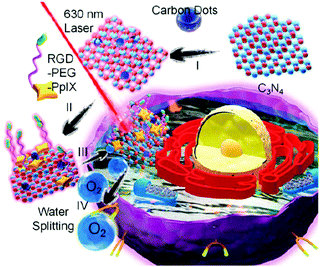 |
| | Fig. 6 Strategy of red light triggered carbon dot doped carbon nitride for intracellular O2 generation via water splitting. Reprinted with permission from Zheng et al.62 Copyright© 2016 American Chemical Society. | |
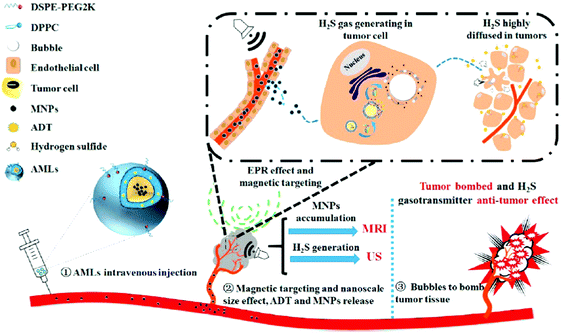 |
| | Fig. 7 H2S donor loaded magnetic liposomes for tumor destruction and real-time MR and US imaging. Reprinted with permission from Liu et al.63 Copyright© 2017 American Chemical Society. | |
2.3. Controllable gas release
The biggest challenge is to control the gas release i.e. the dosage of the administered gas, as the conventional gas administration approach is not feasible in this regard. Uncontrollable gas release leads to non-specific treatment resulting in poor efficiency and potential side effects. Despite using nanocarriers to improve the specificity of gas therapy, it is still necessary to further optimize the strategy by adding the function of trigger-responsive gas release. Ensuing this aspect, various nanoformulations have been designed to release gas molecules upon triggers such as light, acidic environment, ultrasound, magnetic field and enzymes.29–33 The accurate control of gas release via nanocarriers leads to optimal therapeutic results by concentrating the gas in a specific area for short periods.
Controllable gas release upon triggering through chemical reactions, acidic conditions, ultrasound, H2O2 and light is discussed as follows. Phenylboronic acid-containing framboidal NPs modified with CORMs were used for CO delivery to establish anti-inflammatory effects. The CO gas can be released in response to cysteine revealing a simple design for controllable release.65 The H2S gas release via S-aroylthiooxime (SATO) functionalized micelles were used to kill cancer cells. Biologically relevant thiols such as cysteine and glutathione in the cancer cells can diffuse into the core of micelles to react with SATO for H2S generation.66 An enzyme-triggered NO-release strategy was reported recently. For example, a nanoshell of NO donor-modified hyaluronic acid was formulated to encapsulate the dendrimeric prodrug, which contained doxorubicin (DOX) and indocyaninegreen (ICG) as the chemical drug and photothermal agent, respectively. One kind of enzyme, hyaluronidase (HAase), specifically expressed in a tumor microenvironment, can degrade the hyaluronic acid nanoshell for slow release of NO gas. Exposure to an 808 nm laser can further accelerate the NO release, and trigger the DOX delivery from the dendrimeric prodrug, and ICG receiving the light can produce high temperature to damage the cancer cells.67 CaCO3 mineralized NPs showed CO2 generation characteristic that can be activated in an acidic tumor environment (∼pH 5). The CO2 bubbles facilitated enhanced echo signal sources for US diagnosis as well.68 A rattle type superparamagnetic iron oxide-encapsulated mesoporous silica NP (SPION@hMSN) was used as a carrier to load the NO donor, BNN6. The author demonstrated that BNN6 can be decomposed after ultrasound irradiation by the radical-induced effect to generate NO free radicals and NO gaseous molecules.69 In another case, L-arginine (LA) as the NO donor was loaded in the pores of hollow mesoporous silica NPs. Focused ultrasound was used to activate H2O2 in the cancer cells, and thus caused more ROS generation. The ROS has stronger oxidation ability to oxidize LA that led to NO generation showing high efficacy in tumors.70 Albumin-stabilized perfluorocarbon (PFC) nanodroplets provided the outstanding ability of oxygen loading and enabled low-frequency US triggered oxygen generation. The inhalation behavior resulted in continuous O2 supply to PFC nanodroplets. The tumor hypoxia region was reduced by the oxygen supply, thereby improving the efficiency of both PDT and radiotherapy (Fig. 8).71 The photosensitizer, chlorin e6 (Ce6), loaded inside the liposomes was administrated before PFC NPs for PDT performance.
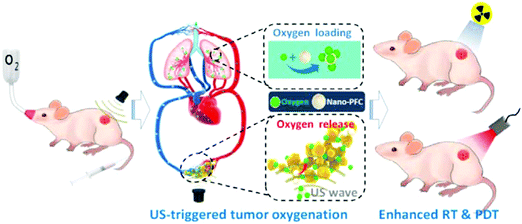 |
| | Fig. 8 Perfluorocarbon (PFC) nanodroplets exhibiting the ability of oxygen loading and low frequency US triggered oxygen release. Reprinted with permission from Song et al.71 Copyright© 2016 American Chemical Society. | |
The cancer cells contain a higher concentration of hydrogen peroxide (50–200 μM, depending on the cell type) than the normal cells (2–8 μM);72,73 therefore, H2O2 can also be utilized as a trigger for gas release from nanocarriers. Jin and co-workers demonstrated H2O2-triggered Mn2(CO)10-loaded hollow mesoporous silica (MnCO@hMSN) NPs for intracellular CO release. The MnCO in hMSN can react with the H2O2 inside the cancer cell to generate hydroxide free radicals through a Fenton-like reaction. And then, the hydroxide free radicals further coordinated with the Mn centre on the MnCO to cause CO release and established CO-induced cytotoxicity (Fig. 9).74 Glucose-responsive sequential generation of H2O2 and NO was demonstrated utilizing a biocompatible/biodegradable hollow mesoporous organosilica NP as a nanocarrier for the co-delivery of glucose oxidase and LA. The glucose oxidase oxidized intratumoral glucose into toxic H2O2 for the subsequent oxidization of LA into NO.75 A similar strategy of glucose-responsive spatiotemporally controlled NO bubble generation was achieved using magnetic microvesicles loaded with glucose oxidase and magnetic NPs.76 A series of H2O2-responsive approaches were reported to achieve controllable release of oxygen. Taking advantage of the high reactivity of manganese dioxide (MnO2) NPs toward endogenous hydrogen peroxide (H2O2) allowed generation of O2 within the tumor microenvironment.77 For example, MnO2 NPs in different formulations, including MnO2–polymer–lipid NPs,78 chlorine e6 (Ce6) loaded MnO2 NPs,77 hyaluronic-coated mannan conjugated MnO2 NPs,79 Au@MnO2 core–shell NPs,80 chlorine e6 (Ce6) and cisplatin prodrug modified albumin-MnO2 NPs,81 and MnO2 nanosheets anchored with upconversion nanoprobes82 all demonstrated the capability to overcome hypoxia to effectively enhance the efficacy of cancer therapy by improving intracellular oxygenation. Prussian blue/MnO2 hybrid NPs facilitate photothermal therapy (PTT), photoacoustic imaging and magnetic resonance imaging.
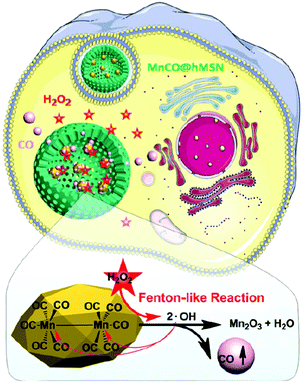 |
| | Fig. 9 H2O2-Triggered MnCO-loaded hMSN NPs producing CO bubbles to cause cytotoxic effect to cancer cells. Reprinted with permission from Jin et al.74 Copyright© 2017 Royal Society of Chemistry. | |
The composition of MnO2 inside the hybrid NPs results in a catalytic reaction with H2O2 for oxygen generation and Mn2+ release, which can be introduced as a H2O2-responsive T1 contrast image. Increasing the oxygen level inside the tumor can not only improve the hypoxia status to inhibit the cancer growth but also enhance the signal intensity of diamagnetic T2-weighted MR imaging by the production of diamagnetic oxygenate hemoglobin that was formed through the binding of deoxygenated hemoglobin with oxygen.83 Two similar cases involved oxygen generation by the reaction of H2O2 and catalase. One of the cases indicated that the catalases were grafted in the open pore channels on mesoporous organosilica NPs. The NPs accumulated in the tumor by the EPR effect to release oxygen bubbles through H2O2 trigger for US imaging, followed by the US-guided high intensity focused US (HIFU) mediated tumor destruction.84 In the other case, hollow PLGA vesicles were used with catalases and methylene blue loaded inside. The surface of the PLGA vesicles was functionalized with a tumor targeting ligand c(RGDfK) to target cancer cells. The intracellular H2O2 can penetrate into the core of the PLGA vesicles through small pores in the PLGA shell that initiated the oxygen generation by the mixing of H2O2 and catalase. The rapid production of O2 disrupted the PLGA vesicles enabling oxygen release inside the cells to enhance the efficiency of PDT. The continuous generation of O2 greatly improved the PDT efficacy in the hypoxic tumor thereby inhibiting tumor growth due to prominent necrosis and destroyed the tumor completely with 7 days of treatment in vivo.85
Photo-responsive strategies find potential applications in non-invasive treatments to achieve remote controlled gas release. Both doxorubicin (DOX) and N,N′-di-sec-butyl-N,N′-dinitroso-1,4-phenylenediamine (BNN6) were loaded in the core of PLGA@PEG micelles. The NO gas can be generated by the decomposition of BNN6 after irradiation with 365 nm light and is released by breaking the shell of the micelles. In this case, NO was demonstrated to reduce the multidrug resistance (MDR) of cancer cells, thereby improving the efficiency of chemotherapy with DOX (Fig. 10).86 An indirect NO release strategy established via UV light triggering was reported; mesoporous silica NPs were used to carry the photo-induced pH jump agent (2-nitrobenzaldehyde, o-NBA) and NO-donors in the pores. And then, the calcium phosphate (CaP) shell as the gatekeeper was coated onto the surface of mesoporous silica NPs to prevent the gas from leaching out. After UV light irradiation, the o-NBA molecules were activated to produce H+ for the creation of an acidic microenvironment leading to rapid degradation of the CaP shell to release NO-donor, thereby yielding NO gas in the physiological environment.87
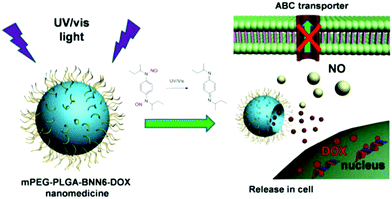 |
| | Fig. 10 Light-triggered PLGA@PEG micelles for NO and DOX release for inhibiting multidrug resistance in cancer cells in situ. Reprinted with permission from Fan et al.86 Copyright© 2016 American Chemical Society. | |
Considering the high tissue penetration, low absorbance by blood and tissues, absence of cell toxicity, and better biosafety, NIR light (800–1300 nm) irradiation is more favorable for medicinal applications compared to UV-vis light. Lanthanide ion doped upconversion NPs (UCNPs) have a specific optical characteristic that makes them absorb light in the NIR range and enables different ranges of UV-vis-NIR light emissions. PhotoCORMs (trans-Mn(bpy)(PPh3)2(CO)2) were modified on the surface of the UCNP@polymer matrix, and 980 nm NIR light was employed to irradiate the NPs for UV-vis light emission, which can trigger the photoCORMs to release CO gas for biological studies (Fig. 11).39 Mn-Carbonyl CORMs caged within graphene oxide nanosheets were used for the photo-chemically activated intracellular release of CO and the subsequent anti-inflammation effect was demonstrated in vitro. The NIR light directly irradiated the graphene oxide to generate active electrons, which can break the bonds of Mn–CO to cause CO gas to leach out from the materials (Fig. 12).88 Prussian blue (PB) NPs have CN− groups and iron atom sites exposed on the outside of the structure for molecular grafting. Iron carbonyl molecules were conjugated with the CN− groups by a ligand exchange process. Realizing that the PB NPs absorb NIR light to transfer heat, the goal of CO release was achieved easily through the heat-induced decomposition of the conjugated iron carbonyl. A large amount of CO was released in the tumor area by the remote NIR light exposure thereby suppressing tumor growth. Owing to the characteristic of light absorption to leach out CO, the PB NPs as CO delivery showed excellent stability and no acute CO-poisoning in vivo (Fig. 13).89 A sandwich structured nanomaterial was synthesized by linking graphene oxide nanosheets and BNN6 via a π–π stacking process. NIR light was irradiated onto the graphene oxides to produce active electrons that can decompose BNN6 for NO release to achieve the anti-cancer effect.90 An NIR responsive NO delivery platform was designed by integrating Roussin's black salt (RBS), a light-sensitive NO donor, with UCNPs to demonstrate on-demand release of NO.91–93 The 980 nm laser excitation of UCNP enabled photo-transfer of NIR-to-UV, which triggered the decomposition of RBS for NO release. Tumor cytotoxicity was observed for higher concentration of NO while anti-multi-drug resistance efficacy was achieved with low concentration of NO together with the chemo-drug as it acted as a potent P-glycoprotein modulator.91 Unlike visible light and NIR, X-ray is a high energy electromagnetic radiation that can be used in medical applications for its advantageous characteristics of high tissue penetrating ability and energy focusing. UCNP@SiO2 (denoted as USMSs) nanoparticles were modified with 3-mercaptopropyltrimethoxysilane (MPTES) to expose the thiol groups. The –SH reacted with tert-butyl nitrite to yield S-nitrosothiol (SNO) on the surface of UCNP@SiO2 nanoparticles. The high energy of X-ray radiation can easily cleave the weak S–N bond of SNO to release NO gas for radio-sensitization thereby enhancing radiotherapy (Fig. 14).94 Various means of remote-controlled gas release are being demonstrated to improve the efficiency, specificity and biosafety. Following this trait, it is feasible to further develop smart nanocarriers for controllable release of gases for future gas therapy applications.
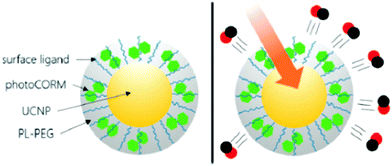 |
| | Fig. 11 Irradiation of a UCNP@polymer matrix within the biological window to release CO. Reprinted with permission from Pierri et al.39 Copyright© 2015 Royal Society of Chemistry. | |
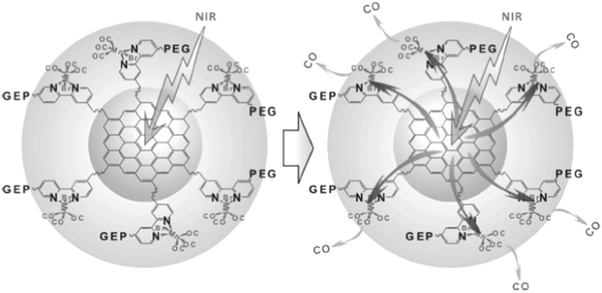 |
| | Fig. 12 NIR light triggered graphene oxide to release CO. Reprinted with permission from He et al.88 Copyright© 2015 Wiley-VCH. | |
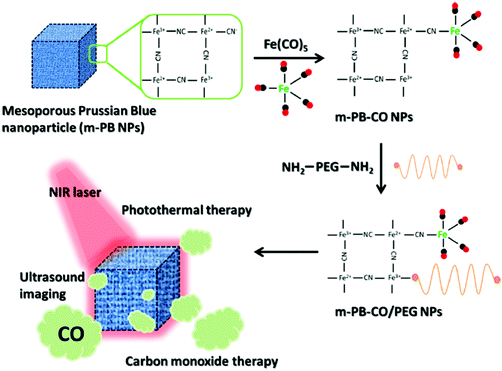 |
| | Fig. 13 Prussian blue NPs carrying iron carbonyl for CO release against malignant tumors upon NIR light exposure. Reprinted with permission from Li et al.89 Copyright© 2016 American Chemical Society. | |
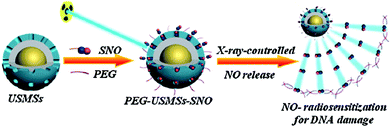 |
| | Fig. 14 UCNP@SiO2 nanoparticles (denoted as USMSs) carrying SNO for NO-enhanced radiotherapy against deep-seated solid tumors upon X-ray exposure. Reprinted with permission from Fan et al.94 Copyright© 2015 Wiley-VCH. | |
2.4. Improving the stability of gas and gas-generation agents
Gases have strong reduction/oxidation characteristics to react easily with bio-species (protein, cell membrane and other bio-molecules) in the surroundings.95,96 It is expected that a lot of gas can be lost by reacting with non-target molecules during the gas delivery process. In addition, gas-generating agents can be unstable making unexpected gas generation in the complicated bio-environment, which contains enzymes and active species. Therefore, gas loss and denaturing of gas-generating agents are the reasons for poor efficiency of gas therapy using the conventional means. In contrast, nanomaterials are appropriate to be used as carriers to increase the stability of gas and gas-generating agents by preventing the contact between them and the environmental substances before reaching the target location.
Some studies have shown the capability of nanocarriers to increase gas stability. Phospholipid based microbubbles were filled with oxygen, and then the microbubbles were coated with saturated diacylphosphatidylcholine and PEG to reveal remarkable stability (half of the original filled oxygen remained after being stored for 3 weeks).97 In general, NO gas and NO donors are very unstable species that pose an immense challenge for NO delivery in bio-systems. Fortunately, different strategies are being developed using nanocarriers to improve the stability of NO gas and NO donors.98–100 For example, a NO donor was loaded in liposomes with tight and loose lipid packing that showed completely different NO gas release kinetics (Fig. 15).99 Besides the lipid packing status, the environmental factors also affected the NO-release behavior. The liposomes encapsulated the NO donor to reveal an extended NO release duration of up to 66 h. However, the NO release behavior significantly changed in the acidic environment at 37 °C; the duration was shortened to 1.3 h. This suggested the potential tumor therapy application through the acid environment-induced NO release.100 NO-releasing poly(lactic-co-glycolic acid)–polyethylenimine NPs (NO/PPNPs) composed of poly(lactic-co-glycolic acid) (PLGA) and polyethylenimine/diazeniumdiolate (PEI/NONOate) were designed for prolonged NO release, antibacterial efficacy, and wound healing activity.101 The SNO-terminated polymers were used to form micelles with the SNO moiety towards the central domain to prevent the contact with the surrounding. The micelle type revealed a longer existence time for SNO that remained because of the prevention of the contact between the embedded SNO and cysteine, compared to the polymer type under the cysteine contained medium at pH 7.4 (Fig. 16).102
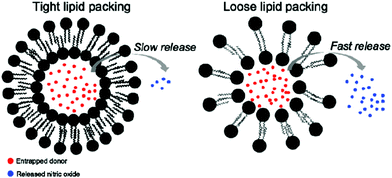 |
| | Fig. 15 Different NO release behaviors observed from NO donor loaded liposomes with tight and loose lipid packing. Reprinted with permission from Suchyta et al.99 Copyright© 2017 American Chemical Society. | |
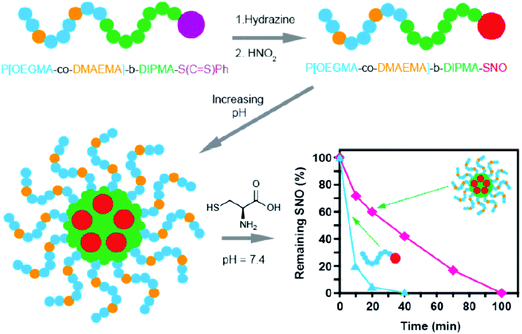 |
| | Fig. 16 Micelle type revealed a longer existence time for SNO compared to the polymer type under the cysteine contained medium, owing to the fact that the micelle type can prevent the contact between the embedded SNO groups and cysteine. Reprinted with permission from Yu et al.102 Copyright© 2015 American Chemical Society. | |
NO donor based silica NPs were synthesized by a sol–gel method using tetraethoxysilane (TEOS) and N-diazeniumdiolate-modified aminoalkoxysilane as precursors that demonstrated prolonged NO release duration (101 h).103S-Nitroso-N-acetyl cysteine encapsulated within silica particles (NAC–SNO NPs) in the polymeric network of a hydrogel synthesized by a sol–gel method could act as a long-lived vasodilator with a sustained activity of trans-S-nitrosating agents. These NAC–SNO NPs exhibited higher efficiency for generating GSNO from glutathione and maintained higher levels of GSNO concentration for a longer time (24 h). The intravenous infusion of the NAC–SNO NPs into hamsters in vivo resulted in a sustained decrease in mean arterial pressure and induced longer vasodilatory effects.104 The NO donor, ruthenium complex cis-[Ru(bpy)2(NO)(4PySH)](PF6)3, termed (Ru-4PySH), was coupled to Au NPs to induce a vasodilator effect by releasing low level of NO in a constant manner, resulting in soluble guanylyl cyclase–cyclic guanosine monophosphate (sGC–cGMP)-mediated processes independent of K+ channel activation. This represents a new pharmacological strategy for the controlled NO release activated by selective biological targets. The coupling of Au NPs with Ru-4PySH has contributed towards reducing the initial large bolus of NO release (burst release). Subsequently, the time-course for the vascular relaxation induced by Au NPs–{Ru-4PySH}n (750 s) also was seen to increase as compared to Ru-4PySH (450 s).105
A photo-responsive polymersome shell with embedded Cu1.6S NPs and GSNO encapsulated inside the polymersome core was designed to enhance the stability of NO donors and prospectively enabled the spatiotemporal regulation of NO release. The reaction between NPs and GSNO as a result of the disintegration of PLGA polymersomes induced by the photothermal effect facilitated NO release, which caused a dramatic increase in the diameter of the basilar artery proving the vasodilation effect (Fig. 17).98 Self-assembled nanofiber gels containing a peptide amphiphile with covalently attached ruthenium tricarbonyl [Ru(CO)3Cl2]2 showed prolonged CO release kinetics compared to the soluble tricarbonylchloro(glycinato)ruthenium (RuCl(gly)(CO)3), which is a kind of carbon monoxide-releasing molecule, to be commonly called CORM-3, alone.106
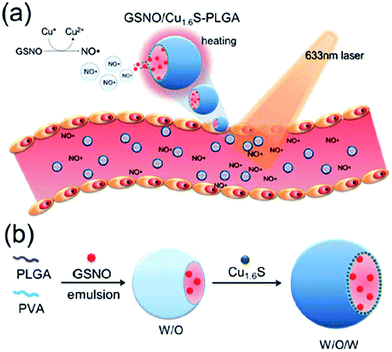 |
| | Fig. 17 (a) Illustration of the vasodilation effect of the polymersome structure upon laser irradiation; (b) polymersome structure for improved stability of the entrapped NO donor. Reprinted with permission from Kao et al.98 Copyright© 2017 Royal Society of Chemistry. | |
3. Therapeutic effect of gas-generating/releasing NPs
In addition to the anti-bacterial, anti-fungal and wound healing properties, NO-releasing NPs also act as an efficient vasodilator.64,98,104,105 The overproduction of ROS is believed to be the foremost detrimental cause in the pathogenesis of I/R injury that leads to inflammation, apoptosis and subsequent tissue damage. Localized therapeutic dosage of CO via CO-releasing NPs shows remarkable anti-inflammatory effect,88 anti-proliferative effect44,46,89,107 and vasorelaxation.45 The hypoxia region in solid tumors hinders the positive effect of various treatment strategies such as chemotherapy, radiotherapy, and photodynamic therapy. One potential way to handle this situation is by supplying oxygen to the tumor hypoxia region in order to improve the effectiveness in the case of treatment resistance in tumors. There have been studies reported recently that enabled the treatments to be more effective by utilizing O2-generating NPs to modulate the unfavorable tumor microenvironment with nanotechnology in order to overcome the current limitations of cancer therapies.62,72–77,80
Although different kinds of NP-based gas delivery strategies have been designed, there are limited pre-clinical studies. Most of the reported literature studies have tried evaluating their system of gas-generating NPs in vitro. There are relatively fewer studies in this scenario of in vivo examination of the therapeutic effect of gas delivery via nanomaterials. Notably, the biological environments of cell studies are quite different from those of animal models or human trials. NO delivery strategies utilizing nanomaterials are dominant in the field of gas therapy compared to the other gases. This reveals that more studies should be conducted on the other therapeutic gases because they also possess beneficial therapeutic properties and cannot be neglected. In this section, we would discuss the therapeutic studies based on the nanocarriers for specifically targeted controlled release of therapeutic gases. The discussion mainly emphasizes the preclinical studies with cell/animal models proving the therapeutic effects rather than the aforementioned nanotechnology for design and mechanisms elucidating gas releasing properties.
3.1. Anti-inflammatory – cardiovascular and wound healing
Oxidatively stressed H9c2 rat cardiomyocytes showed improved viability (50.8%) when treated with a nanofiber gel containing a peptide amphiphile (PA) with CORM-3, demonstrating its potential as a biodegradable gel for localized therapeutic CO delivery (Fig. 18a).106 NIR-responsive nanomedicine (MnCO–graphene oxide (GO)) was constructed by caging Mn-carbonyl CORMs within a small GO nanosheet. The anti-inflammatory effect of MnCO–GO was investigated using Raw264.7 cells as a model cell line and several typical inflammation factors including IL-6, IL-10, IL-12, TNF-α, and nitrite were evaluated to reflect the inflammatory response of Raw264.7 cells. Following the stimulation of lipopolysaccharides (LPS), the levels of IL-6, IL-10, TNF-α, and nitrite were found to increase (Fig. 18b). The treatment with NIR irradiation alone without MnCO–GO nanomedicine and treatment with MnCO–GO nanomedicine in the absence of NIR irradiation did not cause an apparent effect on the inflammation factors. However, the combined treatment of Raw 264.7 with 50 μg mL−1 of MnCO–GO and the NIR light effectively suppressed the levels of IL-6, IL-10, TNF-α, and nitrite, suggesting an obvious anti-inflammation effect of MnCO–GO because of the intracellular release of CO (Fig. 18b).88
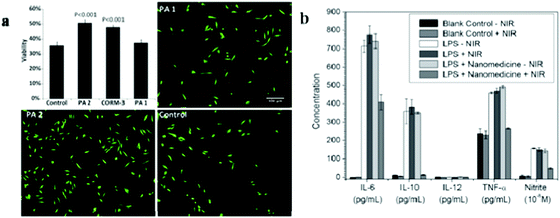 |
| | Fig. 18 (a) Viability of cardiomyocytes after treatment with H2O2 and exposure to CO-releasing peptide amphiphile (PA) contained in nanofiber gels. Fluorescence images of the control group with non-CO-releasing-PA contained in nanofiber gels without CORM-3 (PA1), CO-releasing-PA contained in nanofiber gels with CORM-3 (PA2), and H2O2 negative control are shown, with red and green colors indicating dead cells and live cells, respectively. Reprinted with permission from Matson et al.106 Copyright© 2012 Royal Society of Chemistry. (b) Anti-inflammatory effect of CO released from MnCO–GO nanomedicine under NIR irradiation. LPS (1 μg mL−1) was used to stimulate the inflammatory response of Raw264.7 cells and the absence of LPS and nanomedicine served as the blank control. Reprinted with permission from He et al.88 Copyright© 2015 Wiley-VCH. | |
Prolonged NO release, antibacterial efficacy, and wound healing activity were achieved using NO-releasing poly(lactic-co-glycolic acid)–polyethylenimine NPs (NO/PPNPs) composed of poly(lactic-co-glycolic acid) (PLGA) and polyethylenimine/diazeniumdiolate designed by Nurhasni et al.101 Fast wound healing and epithelialization in a mouse model of an MRSA-infected wound was observed with NO/PPNPs treatment which can be attributed to the bactericidal effect as well as the wound healing activity of NO. A clear epithelialization was observed in the NO/PPNPs-treated group and the wound area was reduced to 25% (P < 0.05), while the untreated group showed a thicker scab without significant reduction in the wound area due to bacterial infections.
3.2. Anti-microbials
NO-releasing NPs were found to be efficient in the acceleration of wound healing process by fibroblast migration and collagen deposition108 with novel anti-bacterial properties that can circumvent antibiotic resistance.109In vitro studies against bacteria have been reported using NO-releasing NPs.51,110 Gram-positive (MRSA, E. faecalis, S. pyogenes, S. aureus, S. epidermidis) and Gram-negative (E. coli, K. pneumonia, P. aeruginosa) bacteria can be effectively killed using NPs with NO-release ability.111 Co-administration of NO with glutathione (GSH) to generate GSNO from the nitrosylation reaction can be even more promising in the inhibition of bacterium growth in vitro.112 NO-releasing NPs are also potentially effective in treating fungal infections caused by C. albicans.51 Based on the work of Friedman et al. 2008, it is believed that the NO-releasing NPs have an anti-microbial effect against bacteria in vivo.113 Martinez et al. demonstrated the use of topically applied NO through NO-np (np stands for nanoparticle), which possessed both antimicrobial and wound-healing properties, to cutaneous methicillin-resistant Staphylococcus aureus (MRSA) skin infections in a murine model.50 The topical application of NO-np enhanced wound healing by the protection of the dermal structural constituents, such as collagen, from degradation.50 As monitored by transmission electron microscopy, unexposed bacteria showed uniform density in cytoplasmic compartments and cell separation by a cross-wall surrounding a highly contrasting splitting system (Fig. 19a). In 1 h after NO-np (termed as NO) treatment, the cross-wall began to disappear (Fig. 19b) and cellular edema was visible after 4 h (Fig. 19c), followed by the destruction of the cell wall architecture in 7 h (Fig. 19d) and the subsequent cell lysis occurred in 24 h (Fig. 19e). In Balb/c mice, the application of NO-np ectopically onto wounds decreased the size of eschar significantly as seen in Fig. 19f and g. On day 7, the size of eschar of the wounds of groups treated with NO-np (NO) was ∼2 mm, whereas the size of eschar of untreated or np-treated wounds reduced to ∼3 mm for the uninfected groups and ∼4.5 mm for the infected-MRSA untreated or np-treated groups. Replicative viability experiments revealed that the NO gas released from diazeniumdiolate NO donor-modified-silica NPs effectively destructed 99% of cells in the biofilms of E. coli, Staphylococcus aureus, Staphylococcus epidermidis and Candida albicans.51
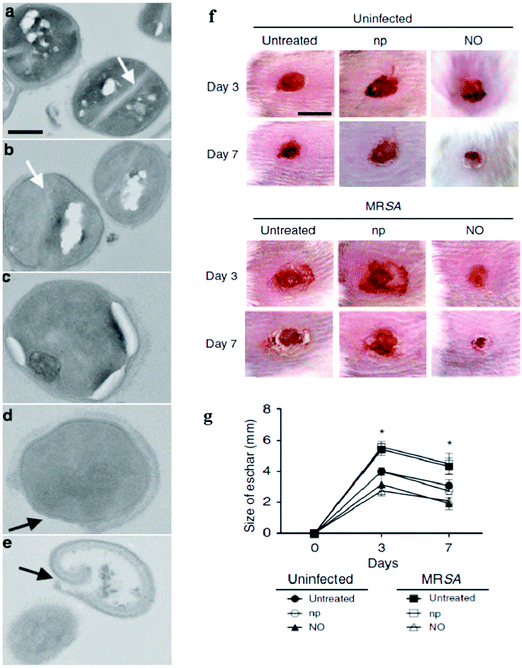 |
| | Fig. 19
Staphylococcus aureus (SA) were exposed to the sustained release of NO by NPs for the evaluation of cell wall damage/lysis and the increase of the wound healing rate when treated with NO-np in mice. (a) Growth of SA in the absence of NO-releasing composites (NO-np) showed an intact cell wall architecture (control). Growth of SA in the presence of NO-np after (b) 1, (c) 4, (d) 7, and (e) 24 h showed increasing destruction of the cell wall architecture, edema, and cell lysis. The white and black arrows denote bacterial cross-wall and cell wall, respectively. Bar = 0.2 μm. (f) Wounds of Balb/c mice for the untreated uninfected-MRSA, uninfected-MRSA treated with np and NO-np shown as NO; untreated infected-MRSA, np-treated infected-MRSA, and NO (NO-np) treated infected-MRSA groups on days 3 and 7. Bar = 5 mm. (g) Wound size analysis of Balb/c mice skin lesions. Time points are the averages of the results for ten measurements. *P < 0.05 in comparing the NO-treated groups with uninfected- or infected-MRSA and untreated groups. Reprinted with permission from Martinez et al.50 Copyright© 2009 Elsevier. | |
3.3. Anti-proliferative – cancer treatments
The water-insoluble and photosensitive CO-releasing molecule, dimanganese decacarbonyl (CORM-1), has been non-covalently embedded into nanoporous non-woven poly(L-lactide-co-D/L-lactide) (PLA) fibers via electrospinning to enable bioavailability and water accessibility of CORM-1. Upon exposure to light (365–480 nm), CO is released from the incorporated CORM-1 and was confirmed by the heterogeneous myoglobin assay. Samples of electrospun 10 wt% and 20 wt% CORM-1 loaded fleece material (CORMA-1-PLA10 and CORMA-1-PLA20, respectively) were used to examine the cell response to high concentrations of CO (Fig. 20a–f). For both samples, the number of dead cells increased with increase in time of illumination (surveyed after 0, 15, and 60 min of irradiation; Fig. 20b). To confirm that truly CO was responsible for the observed cell death, a different setup with non-woven samples in one well and cells in the neighbouring well (Fig. 20c) was used instead of keeping cells and samples in the same well, as used in Fig. 20a. A cover lid over the whole well-plate avoided the loss of the released CO into the atmosphere and allowed the diffusion of CO from the sample to the cells. Fig. 20d shows that more dead cells were found in the cell samples subjected to CO compared to the blank sample demonstrating the toxic effect of the CO gas on 3T3 mouse fibroblasts.46 Light responsive {Re–CO}@Al-MCM-41 mesoporous silica NPs (MSNs) were utilized for CO delivery using photoCORMs that were entrapped within the pores of 100 nm of the mesoporous Al-MCM-41 NPs. In vitro studies using breast cancer cells, MDA-MB-231 cells, proved the cytotoxic effect through the burst of CO upon irradiation (Fig. 20g).44 NIR light-responsive thermal decomposition of CO release was established by the PEGylated iron carbonyl derivatized Prussian blue (PB) NPs. The photothermal conversion upon NIR light irradiation cleaved the Fe–CO coordinating bond, thereby releasing the CO molecules. The combination therapy using CO gas and photothermal treatments showed a synergistic effect against cancer cells by suppressing the tumor growth in vivo without acute CO poisoning (Fig. 20h).89 Hasegawa and Vlies reported polymeric H2S donors (PEG–ADT) based on ADT–OH (ADT: anethole dithiolethione), which altered cellular trafficking of ADT–OH to minimize the unfavorable interactions with intracellular components. It is noted that the amphiphilic donor ADT–OH alone can directly enter the cytoplasm resulting in toxicity (Fig. 20i). The release of H2S from ADT–OH is presumably due to enzymatic reduction that reduced the cell viability as shown in Fig. 20j.114
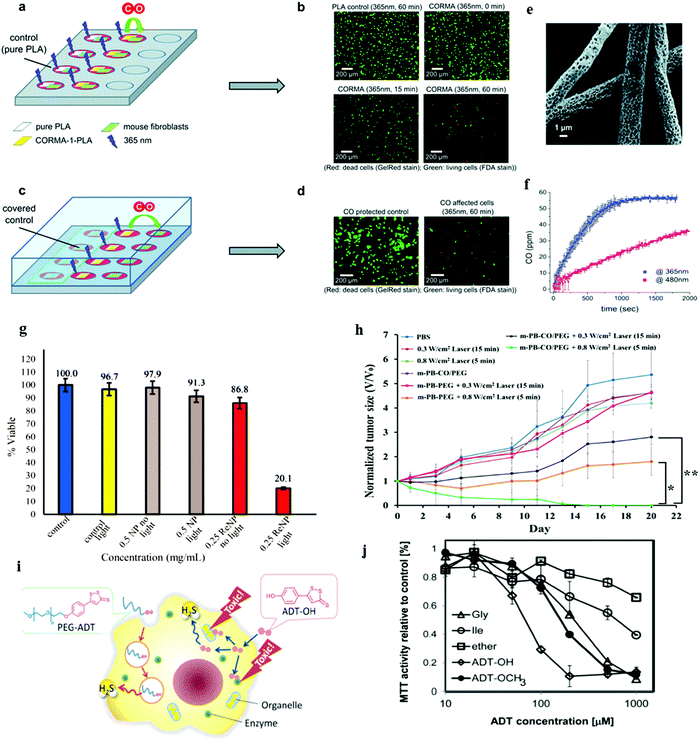 |
| | Fig. 20 Phototoxicity experiments with 3T3 mouse fibroblast cells. (a) Experimental setup where cells and control (left part) or sample (right part) have been illuminated within the well, (b) microscopic images of PLA control (60 min at 365 nm) and CORMA1–PLA-10 (10 wt% of CORM-1) after 0, 15 and 60 min of exposure to UV-A light, (c) experimental setup with separated cells and samples. The released CO gas from the sample can diffuse into uncovered cells (right part), while control cells are protected from CO (left part), (d) microscopic images of a protected control and an unprotected cell sample after exposure of CORMA1–PLA-20 (20 wt% of CORM-1) to UV-A light for 60 min. (e) SEM image of electrospun PLA nanofibrous non-woven CORMA-1–PLA20 (electrospun 20 wt% CORM-1 loaded fleece material) and (f) the corresponding CO release profile upon light exposure. The irradiation of CORMA-1–PLA20 at 365 nm (blue) and 480 nm (red) at an intensity of 10 mW cm−2 showed the wavelength dependency of the CO release rate. Reprinted with permission from Bohlender et al.46 Copyright© 2014 Royal Society of Chemistry. (g) MTT assay of MDA-MB-231 cells. Reprinted with permission from Chakraborty et al.44 Copyright© 2015 American Chemical Society. (h) In vivo antitumor efficacy upon NIR irradiation of PEGylated iron carbonyl derivatized PB NPs. Tumor growth curves were monitored under different treatments (*P < 0.05 and **P < 0.01). Reprinted with permission from Li et al.89 Copyright© 2016 American Chemical Society. (i) Schematic of cellular action of polymeric H2S donor. The polymeric H2S donor PEG–ADT significantly changed the pharmacokinetics and the intracellular trafficking. Only upon hydrolysis, ADT–OH was released slowly from PEG–ADT, which liberated H2S presumably by enzymatic reduction. (j) Cell viability of RAW-Blue cells treated with PEG–Gly–ADT (Gly), PEG–Ile–ADT (Ile), PEG–ADT (ether), ADT–OH, and ADT–OCH3 for 24 h (MTT assay). The hydrolysis rates of the PEG–Gly–ADT and PEG–Ile–ADT conjugates exhibited different toxicity in cells. Reprinted with permission from Hasegawa et al.114 Copyright© 2014 American Chemical Society. | |
A carbon dot doped carbon nitride (C3N4)-based multifunctional nanocomposite (PCCN) for light-driven water splitting was used to increase the intracellular O2 concentration.62 A decrease in the levels of hypoxia-associated proteins CA9 and HIF-α is a hallmark of overcoming hypoxia. As compared with PBS-treated mice, CCN and PCCN treatments decreased the CD31 level by 38% and 24%, respectively, whereas the PpIX treatment increased the CD31 level by 10% (Fig. 21a and b). Notably, PpIX, CCN, and PCN showed little inhibitory effects on 4T1 tumors as seen in Fig. 21c and d. An in vivo study clearly indicated that the PCCN treatment had remarkable tumor growth inhibition with just a single dose of injection through the tail vein, which was attributed to its tumor targeting and O2 generation. The PCCN treatment of mice revealed reduced lung and liver metastasis, as evidenced by the fluorescence imaging in Fig. 21e. Chen et al. have successfully developed a new αvβ3 integrin-targeted, H2O2-activatable, and O2-evolving PDT NP (referred to as HAOP NP) composed of methylene blue (MB) as a photosensitizer, catalase as an O2 evolving agent in the aqueous core, and black hole quencher-3 (BHQ-3) as an ultra-efficient energy quencher of excited photosensitizer in the PLGA polymeric shell, functionalized with a tumor-targeting ligand c(RGDfK) to achieve selective and highly efficient PDT for cancer treatment (Fig. 21f).85 The capability of HAOP NPs to overcome hypoxia in vivo was investigated via hypoxia-inducible factor (HIF)-1α staining assay to evaluate hypoxic conditions in the tumor with or without HAOP NP treatment as shown in Fig. 21g. The immunofluorescence of tumor tissues was very weak for the group treated with HAOP NPs, suggesting that the expression of HIF-1α significantly decreased owing to the generated O2 in the tumor that overcame the hypoxia by the catalase loaded HAOP NPs. The PDT effects were assessed by monitoring the changes of relative tumor volumes (Fig. 21h). After HAOP NP-mediated PDT, tumor growth was completely inhibited, and the tumor was eliminated after 7 day treatment. H&E staining assay was also performed to evaluate the treatment efficacy on the tumors with different treatments. Prominent necrosis was observed in tissue sections from the group treated with HAOP NP-mediated PDT (Fig. 21n), while no obvious necrosis was observed in the tumor tissues from other groups (Fig. 21i–m).
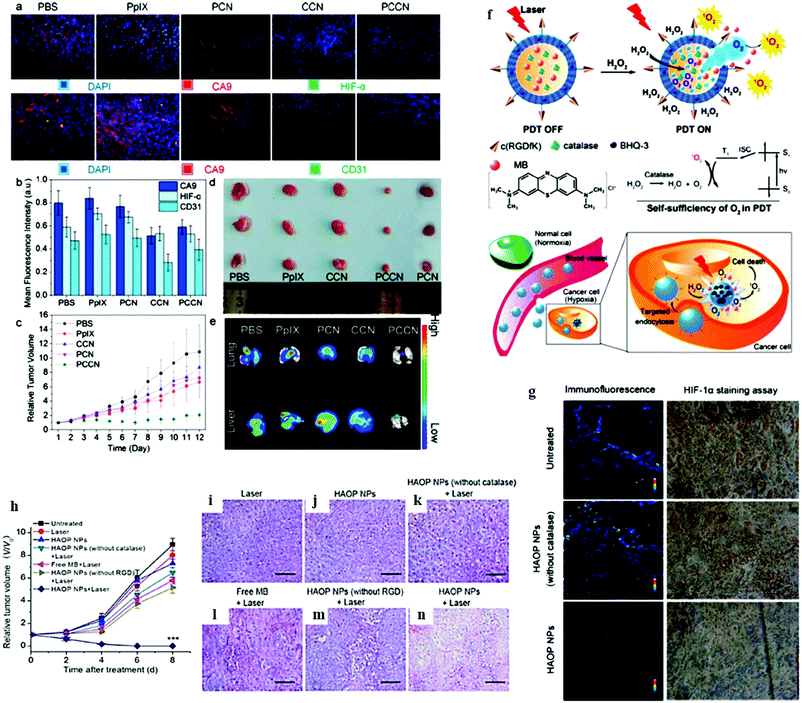 |
| | Fig. 21
In vivo study of overcoming hypoxia and anti-cancer therapy using PCCN in 4T1-tumor-bearing Balb/C mice. (a) Immunofluorescent staining of CA9, HIF-α, and CD31 of tumors, (b) quantitative analysis of CA9, HIF-α, and CD31, (c) the relative tumor volume for the post-treatment, (d) tumor images on the 12th day post-treatment, (e) ex vivo fluorescence imaging of lung and liver metastasis. Reprinted with permission from Zheng et al.62 Copyright© 2016 American Chemical Society. (f) Schematic illustration of H2O2-controllable release of O2 and selective/efficient PDT against hypoxic tumor cells using HAOP NPs. (g) HAOP NPs to overcome tumor hypoxia. Immunofluorescence staining with HIF-1α antibodies and the corresponding HIF-1α staining of tumor slides from U87-MG tumor bearing mice treated with HAOP NPs or HAOP NPs (without catalase) at a dose of 10 mg kg−1. (h) Change of relative tumor volume (V/V0) upon different treatments. Data are means ± SD (n = 6), ***P < 0.001 compared to other groups using one-way ANOVA. (i–n) Tumors at 24 h post-treatment and the corresponding H&E staining of tumor slides. Scale bars: 100 μm. Reprinted with permission from Chen et al.85 Copyright© 2015 American Chemical Society. | |
3.4. Vasodilation
The ruthenium complex cis-[Ru(bpy)2(NO)(4PySH)](PF6)3, termed (Ru-4PySH) which is the NO donor, was coupled to Au NPs so as to modify the NO release profiles, thereby modifying cellular mechanisms without ruining the efficacy. It was found that the time to reach the maximum relaxation was longer for Au NPs–{Ru-4PySH}n (750 s) than for Ru-4PySH (450 s) (Fig. 22a).105 A non-cytotoxic therapeutic vitamin A decorated polymeric NP loaded with GSNO showed efficacious targeting and subsequent, non-systemic, intracellular delivery of NO, down-regulating the profibrogenic activity. The contractile activity of hepatic stellate cells (HSCs) was demonstrated for the potential treatments of both liver fibrosis and portal hypertension without any associated systemic side effects. Portal pressure in bile duct ligated (BDL) rats treated with vitamin A conjugated NO NPs (VA-GSNO-NPs) decreased by about 25% (∼12 mmHg) (Fig. 22b and c).64 A photo-controllable vasodilator GSNO/Cu1.6S PLGA polymersome was demonstrated by Yeh et al. A photo-responsive polymersome shell made of Cu1.6S NPs with GSNO inside the polymersome core was designed to enhance the stability of NO donors and prospectively enabled the spatiotemporal regulation of NO release, thus minimizing the harmful effects associated with conventional NO therapies. Light-induced polymersome rupture caused a dramatic increase in the diameter of the basilar artery (Fig. 22d).98 A photoactive manganese carbonyl complex, namely, fac-[Mn(pqa)(CO)3]ClO4 (abbreviated as {Mn–CO}, pqa = (2-pyridylmethyl)(2-quinolylmethyl)amine) was incorporated into the pores of mesoporous Al-MCM-41 NPs. This {Mn–CO}@Al-MCM-41 rapidly released CO when irradiated with a visible light source (λ > 350 nm). Vasorelaxation could be induced in rat aortic muscle rings through effective release of CO by {Mn–CO}@Al-MCM-41 (Fig. 22e).45
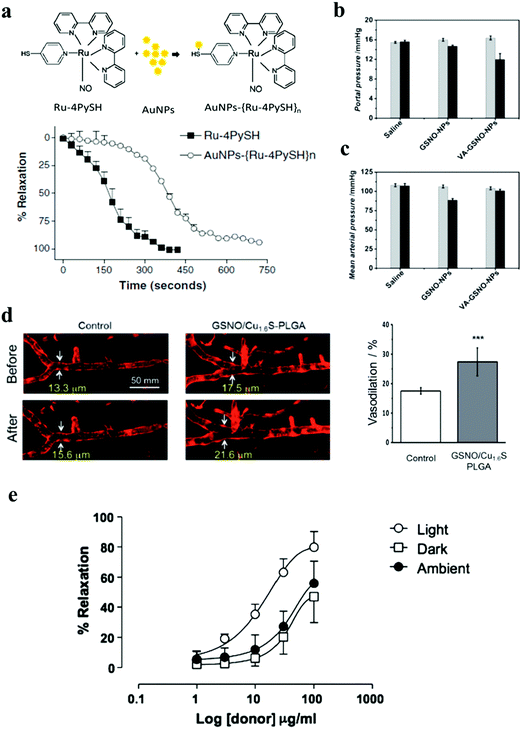 |
| | Fig. 22 (a) Scheme of formation of the Au NPs–ruthenium complex (Au NPs–{Ru-4PySH}n) and time-course for Ru-4PySH and Au NPs–{Ru-4PySH}n-induced relaxation. Denuded thoracic aortic rings were pre-contracted with 0.1 mmol L−1 phenylephrine, and then 0.1 and 5 μmol L−1 of Ru-4PySH or AuNPs–{Ru-4PySH}n were added. Reprinted with permission from Silva et al.105 Copyright© 2014 Springer. (b and c) Evaluation of the portal pressure and arterial pressure in a rat model of cirrhosis. Reprinted with permission from Duong et al.64 Copyright© 2015 Wiley-VCH. (d) Demonstration of the vasodilatory activity of the photo-induced release of NO from GSNO/Cu1.6S PLGA polymersomes on the cerebral blood vessel of larval zebrafish. Quantitative analysis of the fluorescence images shows that the increase of the width of the basilar artery in the zebrafish injected with polymersomes was significantly greater than that of the control (27.4 ± 4.7%), n = 8 vs. 17.5 ± 1.1%, n = 4; p < 0.001. Reprinted with permission from Kao et al.98 Copyright© 2017 Royal Society of Chemistry. (e) Dose-dependent vasorelaxation of rat aortic muscle rings by {Mn–CO}@Al-MCM-41. Reprinted with permission from Gonzales et al.45 Copyright© 2014 Royal Society of Chemistry. | |
4. Conclusions
The earliest development of gas therapy began 130 years ago. Gas therapy was found as a suitable means to solve many tricky medical problems including some acute situations of shock, heart attack, and ischemia-reperfusion injury. It can also be applied as an auxiliary tool to improve the efficacy of the traditional medical treatments. Using gas therapy as preventive and health care medicine is a good choice based on its characteristic functions in delaying aging, reducing the incidence of cardiovascular diseases and cancer, and possible prevention against dementia. However, gas treatments still have some restrictions on clinical applications. The main reasons for those restrictions are poor gas delivery efficiency and uncontrollable gas characteristics. The conventional gas delivery methods are not sufficient for use in future clinical requirements. Therefore, development of a new form of gas therapy using well-designed NPs as carriers is beneficial for medicinal utilization owing to the advantages of stabilizing gas and gas-generation agents so as to transport gases to the specific target, achieving controllable gas release by a feasible trigger, and the promising features of gas loading. The design of nanoformulations has resulted in more specific treatments and better efficiency for curing a variety of diseases compared to conventional gas therapy. Although it is still a challenge to expand the clinical effectiveness of gas therapy through the assistance of a unique nanoformulation, nanomaterials can provide excellent imaging functions such as fluorescence, ultrasound, photoacoustic, light scattering and magnetic resonance imaging that can assist us to understand the detailed gas therapy mechanisms, especially the unknown reasons about how the gases cure the diseases. This kind of research finding can provide very important information to promote the development of novel gas therapy in future. Future advances in gas delivery technology and strategy are expected to augment the efficacy of gas therapy in chronic diseases. It is vital to have additional elaborate research in this scenario, especially clinical trials are necessary to translate preclinical studies that show a positive response in various kinds of disorder. Based on the scope of nanomaterials development, it is highly anticipated that in future the design strategy could be focused on formulating multi-functional nanoparticles that are suitable as novel nanocarriers for gas/gas generating donors as well as provide multi-modal imaging enabling monitoring the treatment in real-time or diagnosis and establishing combination therapy like PDT, PTT, and radiotherapy together with gas therapy. Considering favorable utilization in the clinical field, the prospective nanomaterials design should be improved based on the essential features of the nanocarriers elucidated in this present review, including prevention of gas diffusion, ability to deliver to the specific target, controllable gas release, and improved stability of gas. Compared to the recent loading approaches, new gas or gas donor loading methods should be developed to give better high loading capability. Specific targeting is a very important topic that should be considered for single point treatment through reliable bio-conjugation. Therefore, the most prospective approach to make gas therapy applicable is to design trigger responsive gas releasing nanocarriers that are bio-compatible with high drug loading efficiency, possessing a targeting moiety for active targeting. Precise spatio-temporal control of gas release processes is highly desirable. Developing effective triggers for remote gas release is also needed for non-invasive treatments and curing deep-seated lesions. One of the promising ways is to use X-ray radiation as a potential trigger that should be given more priority in further research as it can penetrate deep into the tissue compared to visible/NIR light. Accordingly, it is highly desirable to develop and fabricate X-ray absorbing nanomaterials such as lanthanide included nanomaterials. There is also a great concern for the biocompatibility of nanocarriers under physiological conditions. The development of biodegradable nanomaterials with gas-controllable capability is indispensable to meet rigorous biological environments. Considering all these aspects and the vast review of literature studies on gas nanocarriers, it is envisaged that all these design notions should be followed to create more unique nanomaterials that can carry medical gases for applications in future gas therapy.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We appreciate the financial support by the Ministry of Science and Technology, Taiwan (MOST 107-2627-M-006-001; MOST 107-2113-M-006-004-MY2). This work was financially supported by the Center of Applied Nanomedicine, the National Cheng Kung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
References
- S. García-Gallego and G. J. Bernardes, Carbon-monoxide-releasing molecules for the delivery of therapeutic CO in vivo, Angew. Chem., Int. Ed., 2013, 53, 9712–9721 CrossRef PubMed.
- B. Wegiel,
et al., Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth, Cancer Res., 2013, 73, 7009–7021 CrossRef CAS PubMed.
- A. S. Almeida, C. Figueiredo-Pereira and H. A. Vieira, Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death, Front. Physiol., 2015, 6, 1–6 CAS.
- C. Peers, Modulation of ion channels and transporters by carbon monoxide: causes for concern?, Front. Physiol., 2012, 3, 1–4 Search PubMed.
- F. Murad, Discovery of some of the biological effects of nitric oxide and its role in cell signaling, Biosci. Rep., 2004, 24, 452–474 CrossRef PubMed.
- P. Lauren,
et al., Nitric oxide mechanism of protectionin ischemia and reperfusion injury, J. Invest. Surg., 2009, 22, 46–55 CrossRef PubMed.
- C. Xu,
et al., The role of hydrogen sulfide in cyclic nucleotide signaling, Biochem. Pharmacol., 2018, 149, 20–28 CrossRef PubMed.
- S. Du, Y. Huang, H. Jin and T. Wang, Protective mechanism of hydrogen sulfide against chemotherapy-induced cardiotoxicity, Front. Pharmacol., 2018, 9, 1–8 CrossRef CAS PubMed.
- F. B. Jensen, Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport, Acta Physiol., 2004, 182, 215–227 CrossRef CAS PubMed.
- K. Cosby,
et al., Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation, Nat. Med., 2003, 9, 1498–1505 CrossRef CAS PubMed.
- M. L. Ellsworth, T. Forrester, C. G. Ellis and H. H. Dietrich, The erythrocyte as a regulator of vascular tone, Am. J. Physiol., 1995, 269, H2155–H2161 CAS.
- J. E. Jagger, R. M. Bateman, M. L. Ellsworth and C. G. Ellis, Role of erythrocyte in regulating local O2 delivery mediated by haemoglobin oxygenation, Am. J. Physiol., 2001, 280, H2833–H2839 CAS.
- Y. Onishi,
et al., Transcutaneous application of carbon dioxide (CO2) induces mitochondrial apoptosis in human malignant fibrous histiocytoma in vivo, PLoS One, 2012, 7, e49189 CrossRef CAS PubMed.
- R. K. Naviaux, Metabolic features of the cell danger response, Mitochondrion, 2014, 16, 7–17 CrossRef CAS PubMed.
- M. P. Schoenfeld, R. R. Ansari, A. Nakao and D. Wink, A hypothesis on biological protection from space radiation through the use of new therapeutic gases as medical counter measures, Med. Gas Res., 2012, 2, 1 CrossRef PubMed.
- D. Nguyen and C. Boyer, Macromolecular and inorganic nanomaterials scaffolds for carbon monoxide delivery: recent developments and future trends, ACS Biomater. Sci. Eng., 2015, 1, 895–913 CrossRef CAS.
- P. T. Burks,
et al., Nitric oxide releasing materials triggered by near-infrared excitation through tissue filters, J. Am. Chem. Soc., 2013, 135, 18145–18152 CrossRef CAS PubMed.
- S. H. Heinemann, T. Hoshi, M. Westerhausen and A. Schiller, Carbon monoxide-physiology, detection and controlled release, Chem. Commun., 2014, 50, 3644–3660 RSC.
- U. Hasegawa, A. J. Van Der Vlies, E. Simeoni, C. Wandrey and J. A. Hubbell, Carbon monoxide-releasing micelles for immunotherapy, J. Am. Chem. Soc., 2010, 132, 18273–18280 CrossRef CAS PubMed.
- B. Jiang,
et al., Mesoporous metallic rhodium nanoparticles, Nat. Commun., 2017, 8, 15581 CrossRef CAS PubMed.
- S. Hernot and A. L. Klibanov, Microbubbles in ultrasound-triggered drug and gene delivery, Adv. Drug Delivery Rev., 2008, 60, 1153–1166 CrossRef CAS PubMed.
- T. K. Nguyen,
et al., Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles, Chem. Sci., 2016, 7, 1016–1027 RSC.
- R. A. Sperling and W. J. Parak, Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles, Philos. Trans. R. Soc., A, 2010, 368, 1333–1383 CrossRef CAS PubMed.
- M. H. Jazayeri, H. Amani, A. A. Pourfatollah, H. Pazoki-Toroudi and B. Sedighimoghaddam, Various methods of gold nanoparticles (GNPs) conjugation to antibodies, Sen. Bio-Sen. Res., 2016, 9, 17–22 CrossRef.
- Z. Xi and B. Zheng, Applications of aptamer-conjugated nanomaterials for targeted cancer therapy, Nanosci. Nanotechnol. Lett., 2018, 10, 309–319 CrossRef.
- N. Gao,
et al., A versatile platform based on black phosphorus nanosheets with enhanced stability for cancer synergistic therapy, J. Biomed. Nanotechnol., 2018, 14, 1883–1897 CrossRef PubMed.
- K. Ulbrich,
et al., Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies, Chem. Rev., 2016, 116, 5338–5431 CrossRef CAS PubMed.
- W. Ge,
et al., Plasmonic-enhanced and Nd3+-sensitized upconversion nanoparticles for magnetically targeted MRI/UCL dual-mode imaging and photothermal therapy, Nanosci. Nanotechnol. Lett., 2017, 9, 416–424 CrossRef.
- M. E. Stewart,
et al., Nanostructured plasmonic sensors, Chem. Rev., 2008, 108, 494–521 CrossRef CAS PubMed.
- Y. T. Chang,
et al., Near-Infrared Light-Responsive Intracellular Drug and siRNA Release Using Au Nanoensembles with Oligonucleotide-Capped Silica Shell, Adv. Mater., 2012, 24, 3309–3314 CrossRef CAS PubMed.
- H. Peng, J. Hu, C. Hu, T. Wu and X. Tian, Microwave absorbing Fe3O4@mTiO2 nanoparticles as an intelligent drug carrier for microwave-triggered synergistic cancer therapy, J. Nanosci. Nanotechnol., 2017, 17, 5139–5146 CrossRef CAS.
- S. Thamphiwatana,
et al., Nanoparticle-stabilized liposomes for pH-responsive gastric drug delivery, Langmuir, 2013, 29, 12228–12233 CrossRef CAS PubMed.
- Q. Liu,
et al., pH-responsive poly(D,L-lactic-co-glycolic acid) nanoparticles with rapid antigen release behavior promote immune response, ACS Nano, 2015, 9, 4925–4938 CrossRef CAS PubMed.
- N. Fomina, C. McFearin, M. Sermsakdi, O. Edigin and A. Almutairi, UV and near-IR triggered release from polymeric nanoparticles, J. Am. Chem. Soc., 2010, 132, 9540–9542 CrossRef CAS PubMed.
- W. Song,
et al., Combined chemo-phototherma therapy of oral carcinoma based on doxorubicin-loaded gold flower nanocomposites, Nanosci. Nanotechnol. Lett., 2018, 10, 1126–1132 CrossRef.
- X. H. Wang, H. S. Peng, W. Yang, Z. D. Ren and Y. A. Liu, Preparation of gold nanoparticles-attached phosphorescent nanospheres for synergistic photothermal and photodynamic therapy, Nanosci. Nanotechnol. Lett., 2017, 9, 227–232 CrossRef.
- Y. Xiao,
et al., Self-assembled nanoparticle mediated survivin-T34A for ovarian cancer therapy, J. Biomed. Nanotechnol., 2018, 14, 2092–2101 CrossRef PubMed.
- R. Ahmad,
et al., Cutting edge protein and carbohydrate-based materials for anticancer drug delivery, J. Biomed. Nanotechnol., 2018, 14, 20–43 CrossRef CAS PubMed.
- A. E. Pierri,
et al., A photoCORM nanocarrier for CO release using NIR light, Chem. Commun., 2015, 51, 2072–2075 RSC.
- E. Grushka and V. Maynard, Measurements of gaseous diffusion coefficients by gas chromatography, J. Chem. Educ., 1972, 49, 565 CrossRef CAS.
- K. M. Kang,
et al., Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors, Med. Gas Res., 2011, 1, 11 CrossRef PubMed.
- B. J. Dixon, J. Tang and J. H. Zhang, The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance, Med. Gas Res., 2013, 3, 10 CrossRef CAS PubMed.
- B. J. Heilman, J. St. John, S. R. Oliver and P. K. Mascharak, Light-triggered eradication of acinetobacter baumannii by means of NO delivery from a porous material with an entrapped metal nitrosyl, J. Am. Chem. Soc., 2012, 134, 11573–11582 CrossRef CAS PubMed.
- I. Chakraborty, S. J. Carrington, J. Hauser, S. R. Oliver and P. K. Mascharak, Rapid eradication of human breast cancer cells through trackable light-triggered CO delivery by mesoporous silica nanoparticles packed with a designed photoCORM, Chem. Mater., 2015, 27, 8387–8397 CrossRef CAS.
- M. A. Gonzales,
et al., Light-triggered carbon monoxide delivery with Al-MCM-41-based nanoparticles bearing a designed manganese carbonyl complex, J. Mater. Chem. B, 2014, 2, 2107–2113 RSC.
- C. Bohlender,
et al., Light-triggered CO release from nanoporous non-wovens, J. Mater. Chem. B, 2014, 2, 1454–1463 RSC.
- A. C. McKinlay,
et al., Nitric oxide adsorption and delivery in flexible MIL-88 (Fe) metal–organic frameworks, Chem. Mater., 2013, 25, 1592–1599 CrossRef CAS.
- S. Marchesini, C. M. McGilvery, J. Bailey and C. Petit, Template-Free Synthesis of Highly Porous Boron Nitride: Insights into Pore Network Design and Impact on Gas Sorption, ACS Nano, 2017, 11, 10003–10011 CrossRef CAS PubMed.
- W. P. Li,
et al., CO2 Delivery To Accelerate Incisional Wound Healing Following Single Irradiation of Near-Infrared Lamp on the Coordinated Colloids, ACS Nano, 2017, 11, 5826–5835 CrossRef CAS PubMed.
- L. R. Martinez,
et al., Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection, J. Invest. Dermatol., 2009, 129, 2463–2469 CrossRef CAS PubMed.
- E. M. Hetrick, J. H. Shin, H. S. Paul and M. H. Schoenfisch, Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles, Biomaterials, 2009, 30, 2782–2789 CrossRef CAS PubMed.
- L. Wang,
et al., Graphene oxide as an ideal substrate for hydrogen storage, ACS Nano, 2009, 3, 2995–3000 CrossRef CAS PubMed.
- Q. Wang, Q. Sun, P. Jena and Y. Kawazoe, Potential of AlN nanostructures as hydrogen storage materials, ACS Nano, 2009, 3, 621–626 CrossRef CAS PubMed.
- D. A. Riccio, J. L. Nugent and M. H. Schoenfisch, Stober synthesis of nitric oxide-releasing S-nitrosothiol-modified silica particles, Chem. Mater., 2011, 23, 1727–1735 CrossRef CAS PubMed.
- N. A. Stasko and M. H. Schoenfisch, Dendrimers as a scaffold for nitric oxide release, J. Am. Chem. Soc., 2006, 128, 8265–8271 CrossRef CAS PubMed.
- Y. He,
et al., Image-Guided Hydrogen Gas Delivery for Protection from Myocardial Ischemia–Reperfusion Injury via Microbubbles, ACS Appl. Mater. Interfaces, 2017, 9, 21190–21199 CrossRef CAS PubMed.
- D. Dobrunz, A. C. Toma, P. Tanner, T. Pfohl and C. G. Palivan, Polymer Nanoreactors with Dual Functionality: Simultaneous Detoxification of Peroxynitrite and Oxygen Transport, Langmuir, 2012, 28, 15889–15899 CrossRef CAS PubMed.
- L. Duan, X. Yan, A. Wang, Y. Jia and J. Li, Highly loaded hemoglobin spheres as promising artificial oxygen carriers, ACS Nano, 2012, 6, 6897–6904 CrossRef CAS PubMed.
- J. Fan,
et al., Synthesis of biocompatible polymeric nanomaterial dually loaded with paclitaxel and nitric oxide for anti-MDR cancer therapy, RSC Adv., 2016, 6, 105871–105877 RSC.
- R. Neha, A. Jaiswal, J. Bellare and N. K. Sahu, Synthesis of surface grafted mesoporous magnetic nanoparticles for cancer therapy, J. Nanosci. Nanotechnol., 2017, 17, 5181–5188 CrossRef CAS.
- M. Song, T. Liu, C. Shi, X. Zhang and X. Chen, Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia, ACS Nano, 2015, 10, 633–647 CrossRef PubMed.
- D. W. Zheng,
et al., Carbon-dot-decorated carbon nitride nanoparticles for enhanced photodynamic therapy against hypoxic tumor via water splitting, ACS Nano, 2016, 10, 8715–8722 CrossRef CAS PubMed.
- Y. Liu,
et al., Magnetic nanoliposomes as in situ microbubble bombers for multimodality image-guided cancer theranostics, ACS Nano, 2017, 11, 1509–1519 CrossRef CAS PubMed.
- H. T. Duong,
et al., The use of nanoparticles to deliver nitric oxide to hepatic stellate cells for treating liver fibrosis and portal hypertension, Small, 2015, 11, 2291–2304 CrossRef CAS PubMed.
- A. J. van der Vlies, R. Inubushi, H. Uyama and U. Hasegawa, Polymeric Framboidal Nanoparticles Loaded with a Carbon Monoxide Donor via Phenylboronic Acid-Catechol Complexation, Bioconjugate Chem., 2016, 27, 1500–1508 CrossRef CAS PubMed.
- J. C. Foster, S. C. Radzinski, X. Zou, C. V. Finkielstein and J. B. Matson, H2S-releasing polymer micelles for studying selective cell toxicity, Mol. Pharmaceutics, 2017, 14, 1300–1306 CrossRef CAS PubMed.
- C. Hu, X. Cun, S. Ruan, R. Liu, W. Xiao, X. Yang, Y. Yang, C. Yang and H. Gao, Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy, Biomaterials, 2018, 168, 64–75 CrossRef CAS PubMed.
- K. H. Min,
et al., pH-controlled gas-generating mineralized nanoparticles: a theranostic agent for ultrasound imaging and therapy of cancers, ACS Nano, 2015, 9, 134–145 CrossRef CAS PubMed.
- Z. Jin, Y. Wen, Y. Hu, W. Chen, X. Zheng, W. Guo, T. Wang, Z. Qian, B. L. Su and Q. He, MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine, Nanoscale, 2017, 9, 3637–3645 RSC.
- K. Zhang, H. Xu, X. Jia, Y. Chen, M. Ma, L. Sun and H. Chen, Ultrasound-triggered nitric oxide release platform based on energy transformation for targeted inhibition of pancreatic tumor, ACS Nano, 2016, 10, 10816–10828 CrossRef CAS PubMed.
- X. Song, L. Feng, C. Liang, K. Yang and Z. Liu, Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies, Nano Lett., 2016, 16, 6145–6153 CrossRef CAS PubMed.
- E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, Boronate-based fluorescent probes for imaging cellular hydrogen peroxide, J. Am. Chem. Soc., 2005, 127, 16652–16659 CrossRef CAS PubMed.
- G. Vilema-Enríquez, A. Arroyo, M. Grijalva, R. I. Amador-Zafra and J. Camacho, Molecular and cellular effects of hydrogen peroxide on human lung cancer cells: potential therapeutic implications, Oxid. Med. Cell. Longev., 2016, 2016, 1908164 Search PubMed.
- Z. Jin, Y. Wen, L. Xiong, T. Yang, P. Zhao, L. Tan, T. Wang, Z. Qian, B. L. Su and Q. He, Intratumoral H2O2-triggered release of CO from a metal carbonyl-based nanomedicine for efficient CO therapy, Chem. Commun., 2017, 53, 5557–5560 RSC.
- W. Fan,
et al., Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy, Angew. Chem., Int. Ed., 2017, 56, 1229–1233 CrossRef CAS PubMed.
- F. Yang, M. Li, Y. Liu, T. Wang, Z. Feng, H. Cui and N. Gu, Glucose and magnetic-responsive approach toward in situ nitric oxide bubbles controlled generation for hyperglycemia theranostics, J. Controlled Release, 2016, 228, 87–95 CrossRef CAS PubMed.
- W. Zhu,
et al., Modulation of hypoxia in solid tumor microenvironment with MnO2 nanoparticles to enhance photodynamic therapy, Adv. Funct. Mater., 2016, 26, 5490–5498 CrossRef CAS.
- C. R. Gordijo,
et al., Design of Hybrid MnO2–Polymer–Lipid Nanoparticles with Tunable Oxygen Generation Rates and Tumor Accumulation for Cancer Treatment, Adv. Funct. Mater., 2015, 25, 1858–1872 CrossRef CAS.
- M. Song, T. Liu, C. Shi, X. Zhang and X. Chen, Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia, ACS Nano, 2015, 10, 633–647 CrossRef PubMed.
- X. Yi,
et al., Core–shell Au@MnO2 nanoparticles for enhanced radiotherapy via improving the tumor oxygenation, Nano Res., 2016, 9, 3267–3278 CrossRef CAS.
- Q. Chen,
et al., Intelligent Albumin–MnO2 Nanoparticles as pH-/H2O2-Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy, Adv. Mater., 2016, 28, 7129–7136 CrossRef CAS PubMed.
- W. Fan,
et al., Intelligent MnO2 Nanosheets Anchored with Upconversion Nanoprobes for Concurrent pH-/H2O2-Responsive UCL Imaging and Oxygen-Elevated Synergetic Therapy, Adv. Mater., 2015, 27, 4155–4161 CrossRef CAS.
- J. Peng,
et al., “One-for-All”-Type, Biodegradable Prussian Blue/Manganese Dioxide Hybrid Nanocrystal for Trimodal Imaging-Guided Photothermal Therapy and Oxygen Regulation of Breast Cancer, ACS Appl. Mater. Interfaces, 2017, 9, 13875–13886 CrossRef CAS.
- T. Liu,
et al., Endogenous Catalytic Generation of O2 Bubbles for In Situ Ultrasound-Guided High Intensity Focused Ultrasound Ablation, ACS Nano, 2017, 11, 9093–9102 CrossRef CAS PubMed.
- H. Chen, J. Tian, W. He and Z. Guo, H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells, J. Am. Chem. Soc., 2015, 137, 1539–1547 CrossRef CAS PubMed.
- J. Fan,
et al., Light-responsive biodegradable nanomedicine overcomes multidrug resistance via NO-enhanced chemosensitization, ACS Appl. Mater. Interfaces, 2016, 8, 13804–13811 CrossRef CAS PubMed.
- H. W. Choi,
et al., Light-induced acid generation on a gatekeeper for smart nitric oxide delivery, ACS Nano, 2016, 10, 4199–4208 CrossRef CAS PubMed.
- Q. He,
et al., NIR-Responsive On-Demand Release of CO from Metal Carbonyl-Caged Graphene Oxide Nanomedicine, Adv. Mater., 2015, 27, 6741–6746 CrossRef CAS PubMed.
- W. P. Li,
et al., Controllable CO Release Following Near-Infrared Light-Induced Cleavage of Iron Carbonyl Derivatized Prussian Blue Nanoparticles for CO-Assisted Synergistic Treatment, ACS Nano, 2016, 10, 11027–11036 CrossRef CAS PubMed.
- J. Fan,
et al., A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO, Nanoscale, 2015, 7, 20055–20062 RSC.
- J. V. Garcia, J. Yang, D. Shen, C. Yao, X. Li, R. Wang, G. D. Stucky, D. Zhao, P. C. Ford and F. Zhang, NIR-triggered release of caged nitric oxide using upconverting nanostructured materials, Small, 2012, 8, 3800–3805 CrossRef CAS PubMed.
- X. Zhang, G. Tian, W. Yin, L. Wang, X. Zheng, L. Yan, J. Li, H. Su, C. Chen, Z. Gu and Y. Zhao, Controllable Generation of Nitric Oxide by Near-Infrared-Sensitized Upconversion Nanoparticles for Tumor Therapy, Adv. Funct. Mater., 2015, 25, 3049–3056 CrossRef CAS.
- P. T. Burks, J. V. Garcia, R. GonzalezIrias, J. T. Tillman, M. Niu, A. A. Mikhailovsky, J. Zhang, F. Zhang and P. C. Ford, Nitric oxide releasing materials triggered by near-infrared excitation through tissue filters, J. Am. Chem. Soc., 2013, 135, 18145–18152 CrossRef CAS PubMed.
- W. Fan,
et al., X-ray radiation-controlled NO-release for on-demand depth-independent hypoxic radiosensitization, Angew. Chem., Int. Ed., 2015, 54, 14026–14030 CrossRef CAS PubMed.
- G. M. Alink, R. M. De Boer, J. Mol and J. H. M. Temmink, Toxic effects of ozone on human cells in vitro, exposed by gas diffusion through teflon film, Toxicology, 1980, 17, 209–218 CrossRef CAS PubMed.
- I. Blumenthal, Carbon monoxide poisoning, J. R. Soc. Med., 2001, 94, 270–272 CrossRef CAS.
- E. J. Swanson, V. Mohan, J. Kheir and M. A. Borden, Phospholipid-stabilized microbubble foam for injectable oxygen delivery, Langmuir, 2010, 26, 15726–15729 CrossRef CAS PubMed.
- P. T. Kao, I. J. Lee, I. Liau and C. S. Yeh, Controllable NO release from Cu1.6S nanoparticle decomposition of S-nitrosoglutathiones following photothermal disintegration of polymersomes to elicit cerebral vasodilatory activity, Chem. Sci., 2017, 8, 291–297 RSC.
- D. J. Suchyta and M. H. Schoenfisch, Controlled release of nitric oxide from liposomes, ACS Biomater. Sci. Eng., 2017, 3, 2136–2143 CrossRef CAS.
- D. J. Suchyta and M. H. Schoenfisch, Encapsulation of N-diazeniumdiolates within liposomes for enhanced nitric oxide donor stability and delivery, Mol. Pharmaceutics, 2015, 12, 3569–3574 CrossRef CAS PubMed.
- H. Nurhasni,
et al., Nitric oxide-releasing poly(lactic-co-glycolic acid)–polyethylenimine nanoparticles for prolonged nitric oxide release, antibacterial efficacy, and in vivo wound healing activity, Int. J. Nanomed., 2015, 10, 3065–3080 CAS.
- S. H. Yu, J. Hu, F. Ercole, N. P. Truong, T. P. Davis, M. R. Whittaker and J. F. Quinn, Transformation of RAFT polymer end groups into nitric oxide donor moieties: en route to biochemically active nanostructures, ACS Macro Lett., 2015, 4, 1278–1282 CrossRef CAS.
- J. H. Shin and M. H. Schoenfisch, Inorganic/organic hybrid silica nanoparticles as a nitric oxide delivery scaffold, Chem. Mater., 2007, 20, 239–249 CrossRef PubMed.
- P. Nacharaju,
et al., A nanoparticle delivery vehicle for S-nitroso-N-acetyl cysteine: sustained vascular response, Nitric oxide, 2012, 27, 150–160 CrossRef CAS PubMed.
- B. R. Silva,
et al., Gold nanoparticle modifies nitric oxide release and vasodilation in rat aorta, J. Chem. Biol., 2014, 7, 57–65 CrossRef PubMed.
- J. B. Matson, M. J. Webber, V. K. Tamboli, B. Weber and S. I. Stupp, A peptide-based material for therapeutic carbon monoxide delivery, Soft Matter, 2012, 8, 6689–6692 RSC.
- G. Dördelmann, H. Pfeiffer, A. Birkner and U. Schatzschneider, Silicium dioxide nanoparticles as carriers for photoactivatable CO-releasing molecules (PhotoCORMs), Inorg. Chem., 2011, 50, 4362–4367 CrossRef PubMed.
- G. Han, L. N. Nguyen, C. Macherla, Y. Chi, J. M. Friedman, J. D. Nosanchuk and L. R. Martinez, Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition, Am. J. Pathol., 2012, 180, 1465–1473 CrossRef CAS PubMed.
- D. O. Schairer, J. S. Chouake, J. D. Nosanchuk and A. J. Friedman, The potential of nitric oxide releasing therapies as antimicrobial agents, Virulence, 2012, 3, 271–279 CrossRef PubMed.
- A. W. Carpenter, D. L. Slomberg, K. S. Rao and M. H. Schoenfisch, Influence of scaffold size on bactericidal activity of nitric oxide-releasing silica nanoparticles, ACS Nano, 2011, 5, 7235–7244 CrossRef CAS PubMed.
- A. Friedman,
et al., Susceptibility of Gram-positive and-negative bacteria to novel nitric oxide-releasing nanoparticle technology, Virulence, 2011, 2, 217–221 CrossRef.
- A. J. Friedman,
et al., Improved antimicrobial efficacy with nitric oxide releasing nanoparticle generated S-nitrosoglutathione, Nitric oxide, 2011, 25, 381–386 CrossRef CAS PubMed.
- A. J. Friedman,
et al., Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites, Nitric oxide, 2008, 19, 12–20 CrossRef CAS PubMed.
- U. Hasegawa and A. J. van der Vlies, Design and synthesis of polymeric hydrogen sulfide donors, Bioconjugate Chem., 2014, 25, 1290–1300 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
|
| This journal is © The Royal Society of Chemistry 2019 |
 a,
Wei-Peng
Li†
ab and
Chen-Sheng
Yeh
a,
Wei-Peng
Li†
ab and
Chen-Sheng
Yeh
 *abc
*abc

























