 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: A siRNA-induced peptide co-assembly system as a peptide-based siRNA nanocarrier for cancer therapy
Wenjun
Li
a,
Dongyuan
Wang
a,
Xiaodong
Shi
a,
Jingxu
Li
a,
Yue
Ma
a,
Yanding
Wang
a,
Tingting
Li
b,
Jianing
Zhang
a,
Rongtong
Zhao
a,
Zhiqiang
Yu
c,
Feng
Yin
*a and
Zigang
Li
*a
aState Key Laboratory of Chemical Oncogenomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Shenzhen, 518055, China. E-mail: lizg@pkusz.edu.cn; Fax: +86-755-2603-3174; Tel: +86-755-2603-3616
bSchool of Advanced Material, Peking University Shenzhen Graduate School, Shenzhen, 518055, China
cSchool of Pharmaceutical Sciences, Guangdong Provincial Key Laboratory of New Drug Screening, Southern Medical University, Guangzhou 510515, China
First published on 6th June 2019
Abstract
Correction for ‘A siRNA-induced peptide co-assembly system as a peptide-based siRNA nanocarrier for cancer therapy’ by Wenjun Li et al., Mater. Horiz., 2018, 5, 745–752.
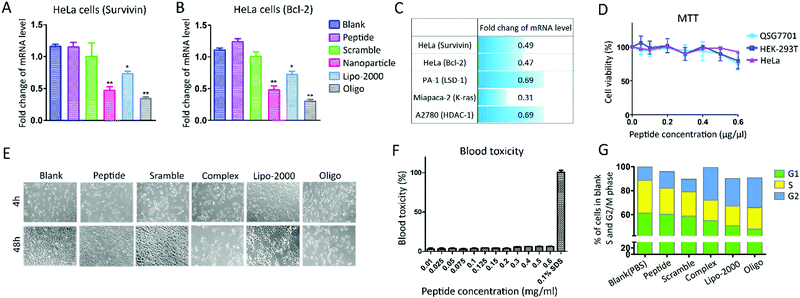
The authors regret an error in Fig. 3E in the originally published manuscript. The correct version of Fig. 3 is shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2019 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)