Highly stable and spectrum-selective ultraviolet photodetectors based on lead-free copper-based perovskites†
Abstract
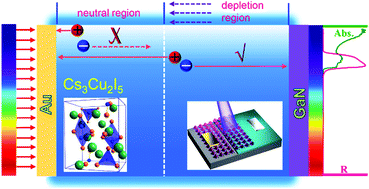
Ultraviolet (UV) photodetectors with high spectrum selectivity are highly desired for certain special applications, like selected-wavelength imaging and UV-phototherapy. Here, for the first time, a highly stable and spectrum-selective self-powered UV photodetector based on lead-free Cs3Cu2I5 films was demonstrated. By designing the Cs3Cu2I5/GaN heterojunction, a narrow spectral response “window” of 300–370 nm was realized, and the working mechanism was investigated through the dependence of the spectral response on the absorber thickness and bias voltage. Because of the high material integrity of the Cs3Cu2I5 films and competent interfacial charge transfer from Cs3Cu2I5 to GaN, the proposed device exhibits a remarkable photodetection capability. Moreover, the proposed photodetector without encapsulation exhibits excellent working stability in ambient air, and typically it can operate continuously for more than 12 h under a high operation temperature of 373 K, indicating a high temperature tolerance and desired performance for practical applications under harsh environments. In addition, the device was used as the sensing pixels in a UV imaging system, and high-resolution imaging patterns were achieved. The results highlight the great potential of such lead-free Cs3Cu2I5 as environment-friendly alternatives for stable and spectrum-selective UV photodetection without band-pass filters that can be employed in imaging.
- This article is part of the themed collection: Materials Horizons Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...