Charge control of fluorescent probes to selectively target the cell membrane or mitochondria: theoretical prediction and experimental validation†
Abstract
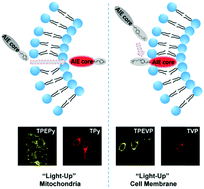
To achieve efficient and precise design of fluorescent probes, unraveling the intrinsic mechanism of their selectivity on different subcellular organelles in cell imaging is important. Using a theoretical protocol combining large-scale molecular dynamics simulations and the hybrid QM/MM model, we explored the mechanism of the strikingly different fluorescence imaging behaviors of two amphiphilic AIEgens with quite similar chemical structures. We proposed that the hydrophobic moiety and the pyridine group of AIEgens manipulate the emission behaviors, and the charged headgroups control the permeation ability through the cell membrane. Therefore, we proposed a molecular design strategy of AIEgen-based fluorescent probes to selectively target the cell membrane or mitochondria, and designed and synthesized four AIEgens. The cell imaging experimental results successfully confirmed the theoretical prediction, which would open an efficient shortcut to design AIEgen-based fluorescent probes to selectively target the cell membrane or mitochondria and lays a solid foundation for the rational design of advanced cell imaging materials.
- This article is part of the themed collection: A selection of 2019 articles


 Please wait while we load your content...
Please wait while we load your content...