Photocatalytic effect of ZnO on the stability of nonfullerene acceptors and its mitigation by SnO2 for nonfullerene organic solar cells†
Abstract
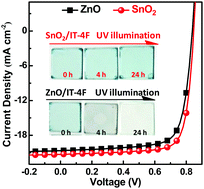
ZnO is the dominantly used electron transporting material in high-performance inverted nonfullerene (NF) organic solar cells. Here, we report that nonfullerene acceptors tend to decompose in the presence of ZnO due to its photocatalytic activity under UV illumination. This leads to poor device stability of NF solar cells under solar light illumination. To mitigate this issue, SnO2 is used as an electron-transporting layer that has a wide band gap and is almost irresponsive to the AM1.5 solar spectrum to replace ZnO. NF solar cells with SnO2 display a power conversion efficiency of 14.1% with the PM6:IT-4F active layer and show better device illumination stability than the reference cells with ZnO.
- This article is part of the themed collection: Materials Horizons 10th anniversary regional spotlight collection: China


 Please wait while we load your content...
Please wait while we load your content...