Nanoarchitectured metal–organic framework/polypyrrole hybrids for brackish water desalination using capacitive deionization†
Abstract
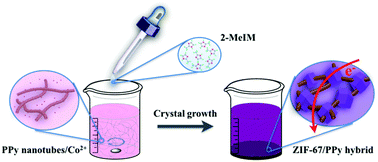
New families of materials that may potentially replace dominant carbon electrodes have emerged as a major research hotspot in the field of capacitive deionization (CDI). Here, we report a metal–organic framework (MOF)/polypyrrole (PPy) hybrid, in which conductive PPy nanotubes that are running through each MOF particle have the potential to increase the overall bulk electrical conductivity, thus promoting such a system as a good CDI electrode material. Consequently, the MOF/PPy hybrid shows a high desalination capacity of 11.34 mg g−1, which is amongst those of state-of-the-art CDI electrodes. Moreover, the MOF/PPy hybrid also shows a superior desalination performance for brackish water and good cycling stability, far exceeding typical carbon-based benchmarks. This is the first example of CDI electrodes derived from direct MOF-based materials, highlighting the potential of these hybrid systems as promising materials beyond traditional carbon electrodes.
- This article is part of the themed collections: Materials Horizons 10th anniversary regional spotlight collection: Asia-Pacific, 2019 Materials Horizons Most Popular Articles and A selection of 2019 articles


 Please wait while we load your content...
Please wait while we load your content...