Phenanthro[9,10-d]triazole and imidazole derivatives: high triplet energy host materials for blue phosphorescent organic light emitting devices†
Muazzam
Idris
 a,
Caleb
Coburn
b,
Tyler
Fleetham
a,
JoAnna
Milam-Guerrero
a,
Peter I.
Djurovich
a,
Caleb
Coburn
b,
Tyler
Fleetham
a,
JoAnna
Milam-Guerrero
a,
Peter I.
Djurovich
 a,
Stephen R.
Forrest
*bcd and
Mark E.
Thompson
a,
Stephen R.
Forrest
*bcd and
Mark E.
Thompson
 *a
*a
aDepartment of Chemistry, University of Southern California, Los Angeles, California 90089, USA. E-mail: met@usc.edu
bDepartment of Physics, University of Michigan, Ann Arbor, Michigan 48109, USA. E-mail: stevefor@umich.edu
cDepartment of Electrical and Computer Engineering, University of Michigan, Ann Arbor, Michigan 48109, USA
dDepartment of Materials Science and Engineering, University of Michigan, Ann Arbor, Michigan 48109, USA
First published on 1st March 2019
Abstract
A class of wide bandgap host materials is introduced as an alternative to carbazole-based hosts to enhance the efficiency and transport properties of organic light emitting diodes (OLEDs). We have synthesized and investigate the photophysical and electrochemical properties of a series of phenanthrene derivatives incorporating fused triazole or imidazole rings. The resulting phenanthro[9,10-d]triazoles and phenanthro[9,10-d]imidazoles are suitable host materials for blue phosphors due to their high triplet energies and conductivities. Incorporation of bulky substituent groups leads to retention of the high triplet energies in the solid state. The most promising materials are incorporated in blue phosphorescent OLEDs that achieve external quantum efficiencies >20%.
Conceptual insightsThere are a limited number of materials with sufficiently high triplet energies to host blue phosphorescent emitters in organic light emitting diodes. Among them, carbazole-based core structures stand out due to their good transport properties. The phenanthro[9,10-d]imidazole core also has a high triplet energy, but green and red emitters using phenanthro[9,10-d]imidazoles hosts are reported to have only moderate efficiencies, and have very low efficiencies when used with blue emitters. The low efficiency is mainly due to low triplet energies of the phenanthro[9,10-d]imidazole in the solid state. Here, we introduce high energy host materials based on both phenanthro[9,10-d]imidazole and phenanthro[9,10-d]triazole cores, using aryl and heteroaryl substituents to retain their high triplet energy in the solid state. The phenanthro[9,10-d]imidazole gives a high blue phosphorescent OLED efficiency. Thus, the phenanthro[9,10-d]imidazole core serves as an alternative to carbazoles for achieving high triplet energies useful as hosts for blue phosphors. |
Introduction
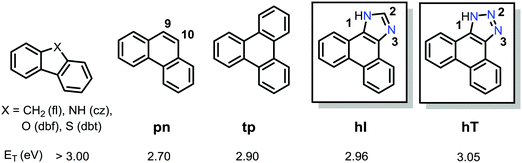
Organic π-conjugated materials have attracted significant attention in the field of optoelectronics, due to their tunable photophysical and electrochemical properties and facile syntheses.1 Understanding the relationship between material structures and their properties is crucial in developing materials with desired photophysical, electrochemical, and thermal properties for application in organic light emitting diodes (OLEDs),2 organic photovoltaics,3 organic field-effect transistors,4 molecular sensors and non-linear optics.5 Over the past few years, OLEDs have attracted considerable attention for full color displays and solid-state lighting.6 This interest is due in large part to the development of transition metal complexes as phosphorescent emitters, making it possible to harvest both singlet and triplet excitons, thereby achieving nearly 100% electroluminescence quantum efficiency.7To achieve high OLED efficiency, a phosphorescent emitter is typically doped into a host matrix to suppress concentration quenching and triplet–triplet/triplet–polaron annihilation, while promoting transfer of charge and/or excited states onto the phosphor.8 The host material requires a triplet energy (ET) higher than that of the phosphorescent dopant to ensure exclusive emission from the dopant. Hosts for green and red phosphors have been developed, leading to highly efficient and long-lived OLEDs. However, there is a dearth of stable host–guest systems with high triplet energies and appropriate frontier orbital energy levels for charge injection and transport for blue OLEDs. In particular, the high triplet energy required for blue OLED hosts limits molecular design to rigid, non-conjugating building blocks with triplet energies approaching 3 eV, such as fluorene, carbazole (cz), dibenzothiophene (dbt) and dibenzofuran (dbf), see Scheme 1. Carbazole-based host materials stand out, due to their high PHOLED efficiencies when combined with blue phosphors.1b,9 In contrast, the triplet energy of phenanthrene (pn, Scheme 1) is too low for this purpose, and thus has only seen limited use in blue host materials.10 However, annulation of a five or six membered ring on the 9,10-positions of the phenanthrene ring increases the triplet energy to levels approaching 3 eV. For example, phenanthro[9,10-d]imidazole (hI) and triphenylene (tp) have triplet energies of up to 2.9 eV. Unfortunately, host materials containing hI and tp cores give only moderate efficiency when paired with green, yellow, red triplet emitters,11 and very low efficiency with blue emitters.12 These poor efficiencies are due to a marked lowering of ET in neat solids relative to their ET in solution. Therefore, we considered whether alternative phenanthrene-cored systems could serve as high triplet energy host materials with high solid-state ET.
In previous work, we used computational methods to screen several phenanthrene derivatives with pyrrole, triazole, imidazole, furan and thiophene fused at the 9,10-positions of phenanthrene. Derivatives with the phenanthro[9,10-d]triazole (hT) and phenanthro[9,10-d]imidazole (hI) cores emerged as structures that have the highest triplet energies.13 Here, we describe a series of phenanthro[9,10-d]triazole and phenanthro[9,10-d]imidazole based materials, and identify structures that have high triplet energies as neat solids. Optimized syntheses of these materials, their photophysical and electrochemical properties, and a strategy to inhibit aggregation induced red-shifts to retain high triplet energies in a neat solid are discussed. The most promising materials are incorporated as host materials for blue phosphorescent OLEDs with >20% external quantum efficiency.
Results and discussion
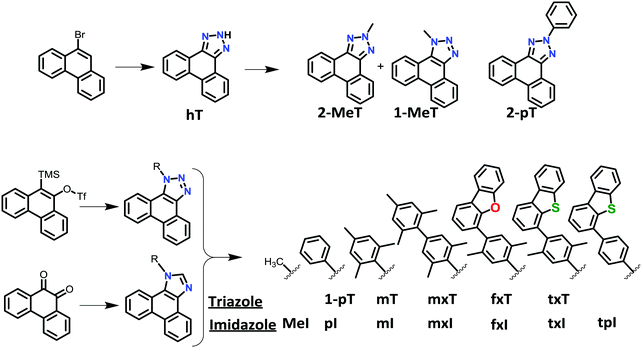
A range of different phenanthro[9,10-d]triazole and phenanthro[9,10-d]imidazole based materials were prepared to investigate the electronic and thermal properties as a function of the substituents on the triazole and imidazole rings (Fig. 1). Alkyl triazoles were prepared from 9-bromo phenanthrene in two steps. The benzyne click chemistry (first step) is reported elsewhere.14 The subsequent alkylation step was carried out in a one pot reaction of hT with K2CO3 and methyl iodide. This reaction yielded predominantly the N2-isomer (2-MeT, 44%) and a minor amount of the N1 isomer (1-MeT, 25%), presumably due to steric interactions between the methyl group and the neighboring proton of the phenanthrene.Aryl-substituted triazoles were prepared using modified procedures of Ueda et al. and Taillefer et al. who reported Ullman conditions for arylation of benzo-triazole.15 Ullman conditions using hT and phenyl halide resulted in 2-pT as the only product in 60% yield. The same result with slightly lower yield was obtained when Buchwald conditions were employed.15a The larger size of the phenyl ring is the likely reason that only the aryl N2 isomer is obtained. Therefore, N1 aryl substituted triazoles were prepared via a modified fluoride-induced elimination to generate benzyne under mild conditions.16 This procedure produced N1 aryl substituted triazoles 1-pT, mT, mxT, fxT and txT in 40–50% yields. A key modification from the literature procedure is preparation of 9-hydroxyphenanthrene using n-BuLi instead of Mg tunings,15a which gives the product in higher yield, with fewer byproducts and shorter reaction times. Phenanthro[9,10-d]imidazoles MeI, pI, mI, mxI, fxI, txI and tpI were prepared from inexpensive starting material (9,10-phenanthrenequinone) from two high yielding steps.12
Targeting materials as blue OLED hosts
The study of the properties of these phenanthrene-based materials started with simple structures, aimed at characterizing the phenanthro-triazole and -imidazole core structures. While our theoretical modeling identified phenanthro-triazole and -imidazole based materials with high triplet energies, they also showed that it was not possible to predict which compounds would make the best host materials without detailed and time consuming modeling in the solid state.13 Moreover, the modeling does not provide the bulk thermal properties of the materials, which is important in making stable OLEDs. Thus, we prepared a number of phenanthro-triazole and -imidazole based materials to identify those structures possessing the desired electronic and thermal properties for viable host materials for blue phosphorescent OLEDs.Absorption spectra of the methyl substituted phenanthro-triazole and -imidazole materials were recorded in 2-MeTHF, Fig. 2a. The lowest energy band of the phenanthro-triazoles are blue-shifted by about 15 nm compared to a similar band of the phenanthro-imidazoles. The properties of the molecules depend on the site of the phenyl substitution. Phenyl substituents at the 1-position of either the triazole- or imidazole-based materials do not lead to a red shift, relative to the methyl substituted analogs (see Fig. S1, ESI†). Steric interactions force the phenyl ring at the 1-position to be out of plane with phenanthro- core and thus disrupts conjugation between the two aromatic ring systems. In contrast, a phenyl substituent at the 2-position adopts a coplanar conformation with the phenanthro[9,10-d]triazole core, leading to conjugation and a red-shift of ca. 30 nm relative to the analogous 1-phenyl substituted compounds. The same red shift has been observed for analogous phenyl substitution on phenanthro[9,10-d]imidazole.13 Thus, substitution at the 1-position in these phenanthro-based materials was chosen to maintain high exciton energy.
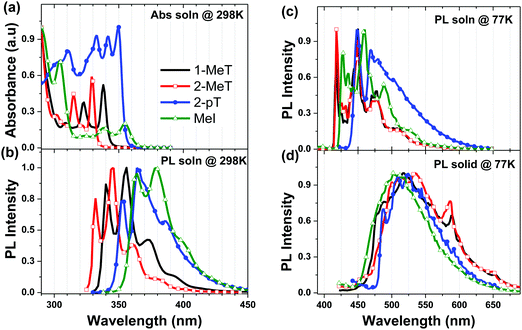 | ||
| Fig. 2 (a) Absorption spectra in 2-MeTHF at 298 K. Normalized emission spectra in 2-MeTHF at 298 K (b) and 77 K (c), and as a neat solid at 77 K (d). | ||
The fluorescent (S1) and phosphorescent (T1) transitions of the triazoles are blue-shifted relative to their imidazole analogs (e.g. compare 1-MeT to MeI, Fig. 2b and c). The triplet energies of 1-MeT and MeI in solution are high (ET = 2.97 and 2.89 eV, respectively); however, the planar structures of 1-MeT and MeI lead to aggregation in thin films, lowering their triplet energies to 2.66 eV and 2.70 eV, respectively (Fig. 2d). The PL efficiency of iridium(III)bis((4,6-di-fluorophenyl)-pyridinato-N,C2′)picolinate (FIrpic), a blue phosphorescent emitter (ET = 2.70 eV),17 doped into a 1-MeT film is low (PLQY < 20%). A higher efficiency is observed for FIrpic doped into MeI (PLQY = 60%), but both hosts fall short of the 80% efficiency observed for FIrpic doped into polystyrene (ET = 3.2 eV).18
Aryl substituents at the 1-position in the triazole and imidazole compounds lead to higher triplet energies in the solid-state (ETsolid) since the out-of-plane conformation of the aryl groups hinders π–π stacking in the condensed phase. The triplet energy of the triazole with a phenyl group at the N1-position (1-pT, ETsolid = 2.75 eV) is higher than that of 1-MeT (ETsolid = 2.67 eV), whereas the values for the imidazole analogs, pI and MeI, are the same (ETsolid = 2.70 eV). To further inhibit aggregation-induced redshifts, mesityl groups were incorporated at the N1-positions of the triazole and imidazole compounds. The resultant materials, mT and mI, have high triplet energies (ETsolid = 2.77 eV and 2.74 eV, respectively) and the PL efficiencies of FIrpic doped into films of both compounds are correspondingly increased (PLQY = 80%).
In addition to maintaining a high triplet energy in the solid state, it is important for a host material to have high sublimation (Ts) and glass transition (Tg) temperature for stable OLED performance. Thermogravimetric analysis showed that mT and mI have Ts < 300 °C, due to their low molecular weight. No Tg was observed for mT, whereas the value for mI is too low (Tg = 72 °C) for OLED applications. Derivatives with a mesityl-o-xylyl group at the N1-position (mxT and mxI) were prepared with increased Ts of 326 °C and Tg of 94 °C. As expected, mxT and mxI have the same energies for the S1 and T1 states (Fig. 3) as mT and mI (Fig. S2, ESI†). The mesityl-o-xylyl group also raises the triplet energy of mxT in the solid-state (ETsolid = 2.77 eV) relative to 1-MeT (ETsolid = 2.67 eV) due to inhibited π–π interactions, although not for mxI relative to MeI (Fig. 3a and b).
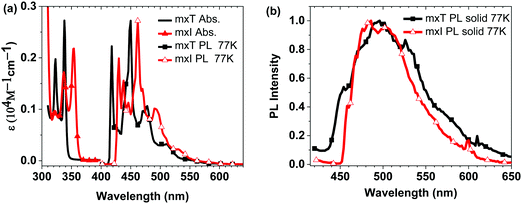 | ||
| Fig. 3 (a) Absorption spectra and normalized emission spectra in 2-MeTHF at 77 K. (b) Normalized emission spectra in neat solid at 77 K. | ||
Dibenzofuran (dbf) and dibenzothiophene (dbt) groups are often used in host materials with high triplet energies. Here, these groups were used to further improve the thermal properties of the phenanthro-triazole and phenanthro-imidazole compounds. The triplet energies for fxT, txT, fxI and txI are similar to mxT and mxI, but the dbf and dbt based compounds have higher sublimation (Ts > 390 °C) and glass transition (Tg = 125–130 °C) temperatures (Table 1). Coupling the dbf and dbt groups to the phenanthro-triazole or -imidazole core through a p-xylyl group proved to be important. If the p-xylyl ring is replaced with a phenyl group, i.e.tpI, conjugation between dbt and phenanthro-imidazole substantially lowers the triplet energy (ETsolid = 2.64 eV). However, placing the p-xylyl spacer between dbt or dbf on fxT, txT, fxI and txI allowed these materials to retain high triplet energies in both solution and the solid-state (ETsolid > 2.75 eV).
| Compound | S1a (eV) | E T (eV) | E ox (V) | E red (V) | HOMO/LUMOe (eV) | T g/Tm/Tsf (°C) | |
|---|---|---|---|---|---|---|---|
| Soln.b | Solidc | ||||||
| a Measured in 2-MeTHF at 298 K. b Measured in 2-MeTHF at 77 K. c Onset of the triplet emission for the neat powder at 77 K. d Obtained from differential pulse voltammetry (DPV) in acetonitrile vs. Fc+/Fc. e Calculated from redox values according to ref. 19. f T g = glass transition temperature, Tm = melting point, Ts = sublimation temperature under nitrogen. | |||||||
| mT | 3.65 | 2.97 | 2.77 | +1.44 | −2.69 | −6.45/−1.66 | —/263/293 |
| mxT | 3.65 | 2.97 | 2.77 | +1.42 | −2.73 | −6.42/−1.61 | 94/247/326 |
| fxT | 3.65 | 2.97 | 2.75 | +1.45 | −2.67, −3.03 | −6.46/−1.68 | 126/237/398 |
| txT | 3.65 | 2.97 | 2.70 | +1.45 | −2.64, −3.00 | −6.46/−1.71 | 128/219/398 |
| mI | 3.45 | 2.89 | 2.74 | +1.00 | −2.96 | −5.94/−1.34 | 72/171/294 |
| mxI | 3.45 | 2.89 | 2.73 | +1.05 | −2.98 | −6.00/−1.31 | 94/196/326 |
| fxI | 3.45 | 2.89 | 2.71 | +1.00 | −2.95, −3.06 | −5.94/−1.35 | 126/—/376 |
| txI | 3.45 | 2.89 | 2.74 | +1.01 | −2.93, −3.04 | −5.95/−1.37 | 130/212/396 |
| mCBP | 3.60 | 2.93 | 2.86 | +0.88 | −2.84 | −5.80/−1.48 | — |
Frontier orbital energies
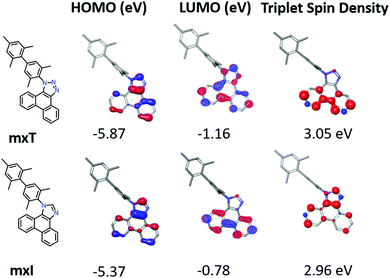
The electrochemical properties of the phenanthro-triazole and -imidazole compounds were characterized using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Data for selected compounds are given in Table 1. Phenanthro-triazoles show irreversible oxidation and quasi-reversible reduction (scan rates of 0.1 V s−1 and 10 V s−1) with the exception of 1-MeT and 2-pT, which show reversible reductions (see Fig. S12, ESI†). Phenanthro-imidazoles have quasi-reversible oxidation and reversible reductions. The phenanthro-triazoles oxidize in the range of 1.42–1.45 V vs. Fc+/Fc, whereas the phenanthro-imidazoles are cathodically shifted by roughly 400 mV (Eox = 1.0–1.05 V). The reduction potentials for the phenanthro-triazoles are similarly shifted by 250 mV relative to those of the imidazole-based materials (Ered = −2.69 and −2.96 V, respectively). The anodic shift for both oxidation and reduction of triazoles relative to their imidazole-based counterparts is due to replacement of carbon with more electronegative nitrogen atom in the triazoles, stabilizing both the HOMO and the LUMO. The HOMO and LUMO energies in Table 1 were estimated from the measured oxidation and reduction potentials.19 The wide HOMO/LUMO gaps of these materials make them suitable for hosting blue phosphorescent dopants such as FIrpic (Eox = 0.92 V, Ered = −2.29 V).20The electronic properties of the phenanthro-triazoles and -imidazoles were also investigated theoretically using density functional and time dependent density functional theory. Geometry optimization in the gas phase was performed using B3LYP functional with an LACVP** basis set. Contours representative for the valence molecular orbitals (MOs) of phenanthro-triazoles and -imidazoles are shown for mxT and mxI in Fig. 4. The HOMO is localized on the phenanthro-riazole/imidazole ring for all compounds, consistent with the small variation in Eox within each phenanthro-triazole and phenanthro-imidazole series. The LUMO is localized on the phenanthro-triazole/imidazole ring for mxT and mxI. In contrast, the LUMO is localized on the dbf moiety in fxT and fxI or dbt moiety in txT and txI whereas, the LUMO+1 is localized on the phenanthro-triazole or -imidazole moiety. The electrochemical data for fxT, fxI, txT and txI, however, suggest that the first reduction occurs on the phenanthro-triazole or -imidazole moiety, and the second reduction on the dbf or dbt moiety (Table 1 and Fig. S13, ESI†). These contrasting results are likely due to similar energies for the calculated LUMO and LUMO+1 of fxT, fxI, txT and txI. (see Fig. S15 and 16, ESI†).
The triplet energies calculated for the phenanthro-triazoles are 3.05 eV and 2.95 eV for phenanthro-imidazoles. These values are similar to the experimental values of 2.97 eV for the triazoles and 2.90 eV for the imidazoles. The triplet spin density for the N1-substituted phenanthro-triazoles and -imidazoles is localized on the phenanthro-triazole/imidazole core (Fig. 4). Exceptions are 2-pT and fxT. In 2-pT the triplet spin density delocalizes over the entire phenyl-phenanthro-triazole, stabilizing the triplet at 2.80 eV (measured = 2.75 eV). The triplet density of fxT is localized on the dbf moiety (Fig. S16, ESI†) due to the similar energies of dbf (T1 = 3.13 eV) and phenanthro-triazole core (T1 = 3.06 eV).
Electroluminescence properties
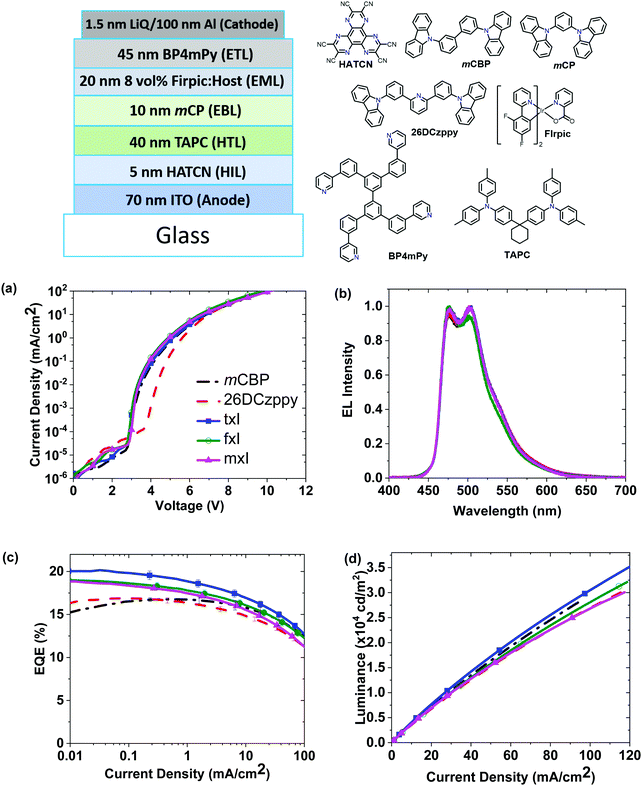
The photophysical, electrochemical and thermal properties of selected phenanthro-triazoles and -imidazoles are summarized in Table 1. All of the listed compounds have suitable electronic properties to host blue phosphorescent OLEDs. The thermal properties of mT and mI are unsuitable for OLED applications, so they were not considered further. Materials with high triplet energies in the solid-state and good thermal properties were incorporated as host materials in blue OLED.In the first set of experiments, a direct comparison was made of OLEDs with phenanthro-triazole and -imidazole hosts, i.e.mxT and mxI, doped with FIrpic (see ESI† for device details and Fig. S17 for device performance). Whereas the triazole and imidazoles based devices exhibit similar current density–voltage (J–V) characteristics and electroluminescence (EL) spectra, devices using mxT hosts degrade rapidly under EL operation, with second and third J–V scans (0.01–100 mA cm−2) showing a >75% drop in efficiency (Fig. S17, ESI†). For comparison the EQE for the imidazole based device drops <10% with the same current cycling. The rapid decrease in efficiency for the mxT based device is likely due to degradation of the phenanthro[9,10-d]triazole host materials, which could be caused by the irreversibility to electrochemical oxidation or reduction. All device testing was done with devices packaged under dry nitrogen. Rapid decay in EQE at high current density is observed with the other phenanthro-triazole devices as well (fxT and txT, Fig. S18, ESI†). It should be noted that this sort of cycling to high current is not a direct indication of the device lifetime, yet the behavior of mxT clearly shows an inherent instability for the triazole based materials. We therefore subsequently focus on imidazole based materials.
In Fig. 5 we compare the performance of OLEDs using the three phenanthro-imidazole based host materials, as well as analogous devices with two conventional host materials (mCBP and 26DCzppy), see Table 2 for device metrics. The device structure is illustrated in Fig. 5. The mCBP and phenanthro-imidazole derivatives have similar J–V characteristics, whereas the 26DCzppy shows a shift to higher voltage, Fig. 5a. The electroluminescence (EL) spectra, shown in Fig. 5b, are independent of the host material and are stable under repeated electrical excitation. The transport properties of these materials were further investigated using hole- and electron-only devices (Fig. S20, ESI†). The J–V characteristics of these single carrier devices are largely independent of the host, with mCBP devices showing 6 ± 3% and 15 ± 4% lower voltages at 10 mA cm−2 for electron- and hole-only devices, respectively, compared to the phenanthro-imidazole host devices. The phenanthro-imidazole and reference hosts devices exhibit similar brightness at low current densities with slight differences at higher current densities (Fig. 5d). OLEDs with the txI host give the highest efficiency (peak EQE = 20.2 ± 0.3%, CE = 43.9 cd A−1), whereas OLEDs utilizing fxI and mxI hosts give slightly lower efficiencies (19%) (Fig. 5c and Table 2). The efficiency of devices employing txI is higher than fxI and mxI is due to higher triplet energy of txI in the solid-state than that of the latter compounds. This could be due to slightly larger size of dbt unit in txI than dbf unit in fxI or mesityl unit in mxI which helps to reduce aggregation-induced redshift of txI in the solid state. The mCBP host device efficiency is marginally lower with a peak EQE = 16.7 ± 0.5% and CE = 39.0 cd A−1.
| Host | PLQYa (%) | EQEmax (%) | V on (V) | CE (cd A−1) | ||
|---|---|---|---|---|---|---|
| @100 cd m−2 | @1000 cd m−2 | @10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 000 cd m−2 |
||||
| a PLQY of spin coated film doped with 10 wt% FIrpic in host, measured using an integrating sphere. b Measured at 1 cd m−2. | ||||||
| mxI | 77 | 19 | 3.2 | 43.1 | 40.3 | 33.2 |
| fxI | 66 | 19 | 3.1 | 42.1 | 39.4 | 33.7 |
| txI | 77 | 20 | 3.1 | 46.8 | 43.9 | 37.2 |
| mCBP | 87 | 17 | 3.3 | 40.2 | 39.0 | 34.9 |
| 26DCzppy | 88 | 17 | 4.1 | 40.2 | 38.1 | 32.4 |
Summary
A series of phenanthro[9,10-d]imidazole/triazole host materials were developed as alternative non-carbazole hosts for blue PHOLEDs. The synthesis, photophysics and electrochemical properties of N2 and N1-aryl substituted phenanthro[9,10-d]triazoles are described. The materials exhibit wide energy gaps and triplet energies as high as 2.97 eV, which are crucial parameters for use as hosts for blue PHOLEDs. The triplet energies undergo a marked redshift of ca. 0.30 eV in the solid state compared to values in solution. To partially overcome aggregation effects, bulky substituent groups were incorporated into the 1-position, reducing the red shift in the solid state to 0.1 eV. The triplet energy in the solid state is sufficiently high to host cyan-emitting FIrpic with a PLQY of near unity. Furthermore, these materials exhibit moderate glass transition temperatures with no decomposition observed during sublimation.The optimized materials were incorporated as host materials for blue OLEDs, showing similar transport properties as mCBP. Devices using phenanthro-triazole host materials exhibited a maximum EQE of 21%, although they were quite unstable. In contrast, devices using phenanthro-imidazole hosts showed a maximum EQE of 20% and a roll-off similar to an mCBP hosted device, indicating improved stability of these materials. Therefore, the phenanthro-imidazole hosts can serve as alternatives to carbazole-based host materials for OLEDs using blue phosphorescent and thermally activated delayed fluorescent (TADF) emitters.
Conflicts of interest
Two of the authors (M. E. T and S. R. F.) have a financial interest in one of the sponsors of this work, i.e. Universal Display Corporation.Acknowledgements
The authors thank the Department of Energy, Office of Energy Efficiency and Renewable Energy (Grant #: DE-EE0007077), US Air Force Office of Scientific Research (Grant #: DE-EE0007626) and the Universal Display Corporation for their financial support of this work.References
- (a) C. W. Tang and S. A. VanSlyke, Organic electroluminescent diodes, Appl. Phys. Lett., 1987, 51(12), 913–915 CrossRef CAS; (b) S.-J. Su, C. Cai and J. Kido, RGB Phosphorescent Organic Light-Emitting Diodes by Using Host Materials with Heterocyclic Cores: Effect of Nitrogen Atom Orientations, Chem. Mater., 2011, 23(2), 274–284 CrossRef CAS; (c) S.-C. Dong, L. Zhang, J. Liang, L.-S. Cui, Q. Li, Z.-Q. Jiang and L.-S. Liao, Rational Design of Dibenzothiophene-Based Host Materials for PHOLEDs, J. Phys. Chem. C, 2014, 118(5), 2375–2384 CrossRef CAS.
- (a) X. Ren, J. Li, R. J. Holmes, P. I. Djurovich, S. R. Forrest and M. E. Thompson, Ultrahigh Energy Gap Hosts in Deep Blue Organic Electrophosphorescent Devices, Chem. Mater., 2004, 16(23), 4743–4747 CrossRef CAS; (b) D. F. O’Brien, M. A. Baldo, M. E. Thompson and S. R. Forrest, Improved energy transfer in electrophosphorescent devices, Appl. Phys. Lett., 1999, 74(3), 442–444 CrossRef; (c) P. E. Burrows, S. R. Forrest, T. X. Zhou and L. Michalski, Operating lifetime of phosphorescent organic light emitting devices, Appl. Phys. Lett., 2000, 76(18), 2493–2495 CrossRef CAS; (d) V. Adamovich, J. Brooks, A. Tamayo, A. M. Alexander, P. I. Djurovich, B. W. D'Andrade, C. Adachi, S. R. Forrest and M. E. Thompson, High efficiency single dopant white electrophosphorescent light emitting diodes, New J. Chem., 2002, 26(9), 1171–1178 RSC.
- (a) C. W. Tang, Two-layer organic photovoltaic cell, Appl. Phys. Lett., 1986, 48(2), 183–185 CrossRef CAS; (b) J. J. Chen, S. M. Conron, P. Erwin, M. Dimitriou, K. McAlahney and M. E. Thompson, High-Efficiency BODIPY-Based Organic Photovoltaics, ACS Appl. Mater. Interfaces, 2015, 7(1), 662–669 CrossRef CAS PubMed.
- (a) M. Kitamura and Y. Arakawa, Current-gain cutoff frequencies above 10 MHz for organic thin-film transistors with high mobility and low parasitic capacitance, Appl. Phys. Lett., 2009, 95(2), 023503 CrossRef; (b) U. Zschieschang, V. P. Bader and H. Klauk, Below-one-volt organic thin-film transistors with large on/off current ratios, Org. Electron., 2017, 49, 179–186 CrossRef CAS.
- (a) M. Alfonso, A. Espinosa, A. Tárraga and P. Molina, Multifunctional Benzothiadiazole-Based Small Molecules Displaying Solvatochromism and Sensing Properties toward Nitroarenes, Anions, and Cations, ChemistryOpen, 2014, 3(6), 242–249 CrossRef CAS PubMed; (b) B. Kulyk, S. Taboukhat, H. Akdas-Kilig, J.-L. Fillaut, M. Karpierz and B. Sahraoui, Tuning the nonlinear optical properties of BODIPYs by functionalization with dimethylaminostyryl substituents, Dyes Pigm., 2017, 137, 507–511 CrossRef CAS.
- (a) T. Tsujimura, New OLED Applications, in OLED Display Fundamentals and Applications, ed. A. C. Lowe and I. Sage, 2017 CrossRef; (b) C. Coburn, C. Jeong and S. R. Forrest, Reliable, All-Phosphorescent Stacked White Organic Light Emitting Devices with a High Color Rendering Index, ACS Photonics, 2018, 5(2), 630–635 CrossRef CAS; (c) F. So, J. Kido and P. Burrows, Organic Light-Emitting Devices for Solid-State Lighting, MRS Bull., 2008, 33(7), 663–669 CrossRef CAS.
- (a) M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Highly efficient phosphorescent emission from organic electroluminescent devices, Nature, 1998, 395, 151 CrossRef CAS; (b) M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Very high-efficiency green organic light-emitting devices based on electrophosphorescence, Appl. Phys. Lett., 1999, 75(1), 4–6 CrossRef CAS; (c) C. Adachi, R. C. Kwong, P. Djurovich, V. Adamovich, M. A. Baldo, M. E. Thompson and S. R. Forrest, Endothermic energy transfer: a mechanism for generating very efficient high-energy phosphorescent emission in organic materials, Appl. Phys. Lett., 2001, 79(13), 2082–2084 CrossRef CAS; (d) C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, Nearly 100% internal phosphorescence efficiency in an organic light-emitting device, J. Appl. Phys., 2001, 90(10), 5048–5051 CrossRef CAS.
- (a) R. J. Holmes, S. R. Forrest, Y. J. Tung, R. C. Kwong, J. J. Brown, S. Garon and M. E. Thompson, Blue organic electrophosphorescence using exothermic host–guest energy transfer, Appl. Phys. Lett., 2003, 82(15), 2422–2424 CrossRef CAS; (b) S. Tokito, T. Iijima, Y. Suzuri, H. Kita, T. Tsuzuki and F. Sato, Confinement of triplet energy on phosphorescent molecules for highly-efficient organic blue-light-emitting devices, Appl. Phys. Lett., 2003, 83(3), 569–571 CrossRef CAS.
- (a) C. W. Lee and J. Y. Lee, Above 30% External Quantum Efficiency in Blue Phosphorescent Organic Light-Emitting Diodes Using Pyrido[2,3-b]indole Derivatives as Host Materials, Adv. Mater., 2013, 25(38), 5450–5454 CrossRef CAS PubMed; (b) S. Y. Byeon, J. H. Kim and J. Y. Lee, CN-Modified Host Materials for Improved Efficiency and Lifetime in Blue Phosphorescent and Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes, ACS Appl. Mater. Interfaces, 2017, 9(15), 13339–13346 CrossRef CAS PubMed; (c) M. M. Rothmann, S. Haneder, E. Da Como, C. Lennartz, C. Schildknecht and P. Strohriegl, Donor-Substituted 1,3,5-Triazines as Host Materials for Blue Phosphorescent Organic Light-Emitting Diodes, Chem. Mater., 2010, 22(7), 2403–2410 CrossRef CAS.
- Y.-H. Chen, H.-H. Chou, T.-H. Su, P.-Y. Chou, F.-I. Wu and C.-H. Cheng, Synthesis and photo- and electroluminescence properties of 3,6-disubstituted phenanthrenes: alternative host material for blue fluorophores, Chem. Commun., 2011, 47(31), 8865–8867 RSC.
- (a) H. Huang, Y. Wang, S. Zhuang, X. Yang, L. Wang and C. Yang, Simple Phenanthroimidazole/Carbazole Hybrid Bipolar Host Materials for Highly Efficient Green and Yellow Phosphorescent Organic Light-Emitting Diodes, J. Phys. Chem. C, 2012, 116(36), 19458–19466 CrossRef CAS; (b) K. Togashi, T. Yasuda and C. Adachi, Triphenylene-based Host Materials for Low-voltage, Highly Efficient Red Phosphorescent Organic Light-emitting Diodes, Chem. Lett., 2013, 42(4), 383–385 CrossRef CAS.
- S. Chen, Y. Wu, Y. Zhao and D. Fang, Deep blue organic light-emitting devices enabled by bipolar phenanthro[9,10-d]imidazole derivatives, RSC Adv., 2015, 5(88), 72009–72018 RSC.
- M. R. Daniel Sylvinson, H. F. Chen, L. M. Martin, P. J. G. Saris and M. E. Thompson, Rapid Multiscale Computational Screening for OLED Host Materials, ACS Appl. Mater. Interfaces, 2019, 11(5), 5276–5288 CrossRef PubMed.
- (a) G. Yasuda and H. Kimoto, Substitution reactions of phenanthro[9,10-d]triazole with benzyl chlorides, J. Heterocycl. Chem., 2009, 35(2), 365–369 CrossRef; (b) A. A. Taherpour and M. Faraji, One-pot Microwave-Assisted Synthesis of 1H-Phenanthro[9,10-d][1,2,3]triazole, Molbank, 2008, 2008(3), M577 CrossRef; (c) N. S. Mani and A. E. Fitzgerald, A Step-Economical Route to Fused 1,2,3-Triazoles via an Intramolecular 1,3-Dipolar Cycloaddition between a Nitrile and an in Situ Generated Aryldiazomethane, J. Org. Chem., 2014, 79(18), 8889–8894 CrossRef CAS PubMed.
- (a) S. Ueda, M. Su and L. Buchwald Stephen, Highly N2-Selective Palladium-Catalyzed Arylation of 1,2,3-Triazoles, Angew. Chem., Int. Ed., 2011, 50(38), 8944–8947 CrossRef CAS PubMed; (b) M. Taillefer, N. Xia and A. Ouali, Efficient Iron/Copper Co-Catalyzed Arylation of Nitrogen Nucleophiles, Angew. Chem., Int. Ed., 2007, 46(6), 934–936 CrossRef CAS PubMed.
- Q. Chen, H. Yu, Z. Xu, L. Lin, X. Jiang and R. Wang, Development and Application of O-(Trimethylsilyl)aryl Fluorosulfates for the Synthesis of Arynes, J. Org. Chem., 2015, 80(13), 6890–6896 CrossRef CAS PubMed.
- A. F. Rausch, M. E. Thompson and H. Yersin, Blue Light Emitting Ir(III) Compounds for OLEDs – New Insights into Ancillary Ligand Effects on the Emitting Triplet State, J. Phys. Chem. A, 2009, 113(20), 5927–5932 CrossRef CAS PubMed.
- G. A. George and D. K. C. Hodgeman, Quantitative phosphorescence spectroscopy of polystyrene during photo-degradation and the significance of in-chain peroxides, Eur. Polym. J., 1977, 13(1), 63–71 CrossRef CAS.
- J. Sworakowski, J. Lipiński and K. Janus, On the reliability of determination of energies of HOMO and LUMO levels in organic semiconductors from electrochemical measurements. A simple picture based on the electrostatic model, Org. Electron., 2016, 33, 300–310 CrossRef CAS.
- E. Baranoff, B. F. E. Curchod, F. Monti, F. Steimer, G. Accorsi, I. Tavernelli, U. Rothlisberger, R. Scopelliti, M. Grätzel and M. K. Nazeeruddin, Influence of Halogen Atoms on a Homologous Series of Bis-Cyclometalated Iridium(III) Complexes, Inorg. Chem., 2012, 51(2), 799–811 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and methods. Photophysical data of the host materials. Cyclic voltammetry curves, differential pulse voltammetry, calculated frontier molecular orbitals, DSC and TGA heating curves of the host materials. Hole and electron only J–V characteristics of FIrpic devices with the host materials. See DOI: 10.1039/c9mh00195f |
| This journal is © The Royal Society of Chemistry 2019 |