Open Access Article
Open Access ArticlePhotothermal effect by NIR-responsive excretable ultrasmall-in-nano architectures†
Domenico
Cassano‡
 *ab,
Melissa
Santi‡
*ab,
Melissa
Santi‡
 a,
Francesca
D’Autilia‡
ac,
Ana Katrina
Mapanao
ab,
Stefano
Luin
a,
Francesca
D’Autilia‡
ac,
Ana Katrina
Mapanao
ab,
Stefano
Luin
 b and
Valerio
Voliani
b and
Valerio
Voliani
 *a
*a
aCenter for Nanotechnology Innovation@NEST, Istituto Italiano di Tecnologia, Piazza San Silvestro, 12-56126, Pisa, Italy. E-mail: valerio.voliani@iit.it
bNEST-Scuola Normale Superiore, Piazza San Silvestro, 12-56126, Pisa, Italy. E-mail: domenico.cassano@sns.it
cUnit of Advanced Optical Microscopy, Humanitas Clinical and Research Center, Rozzano, Milan, Italy
First published on 8th March 2019
Abstract
Photothermal therapy (PTT) is a promising (co)treatment with translation potentiality in oncology. Nowadays, the plasmonic nanoparticle-mediated photothermal effect (PT) relies on two well established NIR-responsive platforms: gold nanorods and nanoshells. Nonetheless, these nanostructures are affected by: (i) re-shaping after irradiation that prevents multiple PT treatments, and (ii) severe limitations to clinical translation due to metal persistence issues. Furthermore, evaluation of nanoparticle performance is usually accomplished in vitro or in mouse models, reducing the translational potential of the findings. Here, we report both the straightforward production of narrow-NIR-absorbing gold ultrasmall-in-nano architectures (tNAs) and their suitability as platforms for PT upon CW-irradiation at 808 nm. PT efficiency is fully assessed against 2D cell cultures and customized 3D pancreatic adenocarcinoma models. Remarkably, the morphology of the tNAs is not affected by laser irradiation, allowing for repeated PT cycles and preserving their ability to avoid long-term body persistence in excretory system organs.
Conceptual insightsThe photothermal (PT) effect (i.e. light-to-heat non-radiative conversion) by gold nanoparticles (NPs) is a promising powerful tool for co-treatment of cancer diseases. Despite its unquestionable success in preclinical research, no noble metal PT transducer has obtained approval for clinical use either because: (i) NIR-absorption occurs for gold NPs whose size is above the renal excretion threshold or (ii) anisotropic ultrasmall gold NPs undergo reshaping after PT transduction. Herein, we demonstrate that narrow-NIR-responsive thermo nano-architectures (tNAs) bear the optimal size for renal excretion and sustain repeated series of NIR light-to-heat transduction without losing their functionalities, avoiding metal sintering or reshaping. Having addressed two key hurdles that currently hinder the clinical translation of PT therapy, this work provides a straightforward resolution for unleashing the potential of noble metals in cancer theranostics. |
Introduction
Photothermal therapy (PTT) is a promising technique for non-invasive cancer treatment relying on the induction of tissue hyperthermia (HT), i.e. a localized temperature rising over 40 °C, by the conversion of light to heat.1 An elegant approach to achieve a potential clinically relevant photothermal effect (PT) is through laser irradiation of noble metal nanoparticles (NPs) with localized surface plasmon resonances (LSPRs) in the first biological window (650–950 nm).2 Commonly, NPs that exhibit LSPRs at near-infrared (NIR) frequencies are gold anisotropic structures, which include nanostars and nanorods.2–5 Noticeably, also gold nanoshells have emerged as an excellent photothermal transducer, as their NIR optical response can be easily tuned by adjusting the diameter-to-shell thickness ratio.6 However, despite the excellent light-to-heat conversion performances demonstrated by these nanoplatforms, only nanoshells have reached clinical trial evaluation, whereas nanorods and nanostars are still confined to the preclinical stage.7 The failure of clinical translation of NP-mediated PTT is mainly ascribed to body persistence concerns.8 Indeed, the optical response of nanostars and nanoshells can be tuned in the NIR region at the expense of an increase in NP diameter up to 150 nm.6 On the other hand, body excretion of exogenous materials above 10 nm occurs through the hepatobiliary route that in the case of non-biodegradable noble metals is slow and inefficient.9,10 As also communicated by the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR), exogenous materials for healthcare have to be completely cleared from the body in a reasonable amount of time, avoiding persistence in the organism that would increase the likelihood of toxicity.10 A commonly adopted approach to avoid metal persistence is to reduce NPs size below the threshold for renal clearance, i.e. ultrasmall nanoparticles (USNPs).11 Furthermore, the maximum light-to-heat transduction is obtained by NPs smaller than 5 nm, as the photothermal conversion efficiency is size-dependent.12 Unluckily, LSPRs of excretable gold USNPs are in the UV/visible region, severely limiting their potential application in PTT as they are far from the first biological windows. In this regard, gold nanorods can partially avoid this issue. Indeed, at least one of their dimensions can be tailored in the ultrasmall range, suitable for renal excretion, while maintaining the LSPRs in the NIR.13 However, gold nanorods re-shape into spheres during laser irradiation, preventing the possibility of multiple PTT series and resulting in non-excretable structures.14–16 Strategies to preserve the nanorod morphology, such as silica coating, have been successfully adopted, albeit increasing their size.17Here, we present a straightforward approach to combine body excretion of metals with NIR-triggered PTT by employing ultrasmall-in-nano architectures composed of metal USNPs embedded in biodegradable silica nanocapsules.18,19 Based on this approach, our group has recently demonstrated the massive production of gold passion fruit-like nano-architectures (NAs) and their employment as biodegradable nanoplatforms for dual photoacoustic/ultrasound imaging and drug delivery (some routine characterizations are reported in Fig. S1, ESI†).20–23 Their biodegradation to clearable building blocks has been observed in a number of biological environments in less than 48 h, including full human serum, human blood and cell cultures (Fig. S2, ESI†). Moreover, NAs’ remarkable biocompatibility has been recently demonstrated in vertebrate models together with the daily quantitative evaluation of metal excretion by renal and hepatobiliary routes following biodegradation.24,25 In particular, our group has recently demonstrated that biodegradable NAs can avoid the persistence of gold in organisms due to the interesting excretion rate of the building blocks from both renal and biliary pathways.25
So far, we have also demonstrated the versatility of the production protocol by the composition of NAs comprising USNPs of several noble metals and metal oxides.26,27 Herein, we report the production of biodegradable and excretable narrow-NIR-responsive ultrasmall-in-nano architectures (thermo-NAs, tNAs) and demonstrate their behaviors as optimal platforms for PTT. Notably, while tNAs bear a substantially different optical behavior with respect to standard NAs, their production requires only a subtle modification to the original protocol. This is one of the most important points of strength of our approach, as the robustness of the synthetic protocols is a key-criterion for translatable nanotheranostics. Finally, the PT efficiency and HT efficacy of tNAs is fully-assessed on 2D cell cultures and significant 3D customized models of human pancreatic ductal adenocarcinoma (PDAC), remarkably enhancing their potentiality of translation from the pre-clinical to clinical investigation stage.
Results and discussion
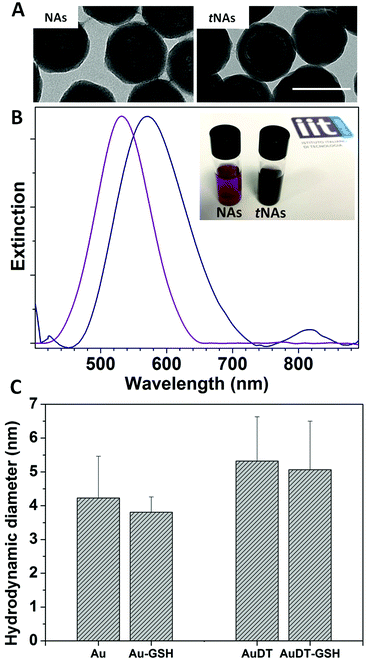
Narrow-NIR-responsive tNAs displaying an overall diameter of 124.3 ± 23.0 nm were synthesized by employing slight modifications to the standard protocol for the production of NAs.20 Anionic gold USNPs were synthesized by fast reduction of an aqueous solution of chloroauric acid by sodium borohydride in the presence of poly(sodium 4-styrene sulfonate) (PSS). USNPs were partially aggregated by adding a DMF solution of (4,4′-dimercaptostilbene) (DMSB) and, finally, a controlled aggregation was obtained by adding an aqueous solution of cationic poly(L-lysine) (PLL). A 20 nm thick silica shell was then built on the USNP-polymer template by a modified Stöber process.20 A typical wide-area TEM image of the tNAs is provided in Fig. S3 (ESI†), together with their size histograms. Noticeably, the entire protocol requires less than 4 h for the production of 15 mg of tNAs from a single operator, and the nanoplatforms have a value of about 1 € per mg by considering the raw materials. ICP-MS quantification on both NAs and tNAs revealed a slight difference in the amount of gold USNPs loaded in the nano-architectures, likely ascribed to the presence of DMSB in the tNAs. In particular, the gold loading, expressed as %w/w, is 4.9% and 3.8% for, respectively, NAs and tNAs. On the other hand, TEM images of tNAs reveal that they have the same overall morphology of standard NAs (Fig. 1A), whereas their optical behaviors differ substantially. The addition of DMSB induces a redshift of the main LSPR in the visible window from 530 to 570 nm in tNAs, and causes the appearance of another plasmonic band in the NIR region (Fig. 1B, background-subtracted extinction spectra).We then performed DLS measurements on gold USNPs before the encapsulation in NAs and tNAs, in order to confirm their suitability for renal excretion. We found that both in the presence and absence of dithiol moieties, gold USNPs display a hydrodynamic diameter (HD) lower than 6 nm, (Fig. 1C). It is worth to notice that, upon biodegradation of the nano-architectures and the release of gold USNPs, the latter are likely coated by endogenous glutathione (GSH). Hence, we performed DLS measurements in a solution of GSH at a typical intracellular concentration (5 mM). We observed a slight decrease of the HD (Fig. 1C) in a few minutes, which remains in the ultrasmall range for both samples, confirming the suitability for renal excretion of USNPs.
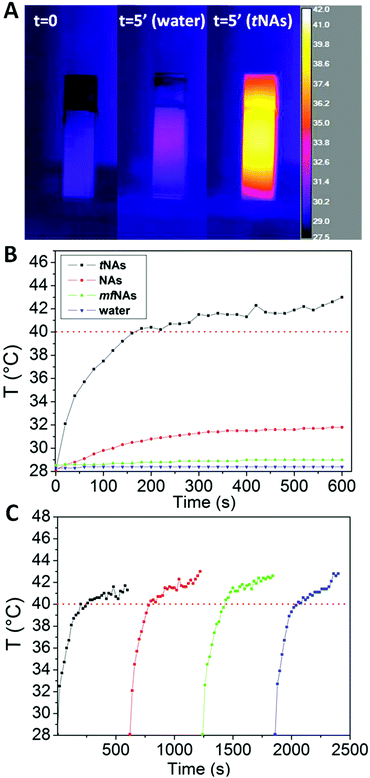
Photothermal transduction efficiency was assessed by measuring the temperature increment of milliQ water-dispersed tNAs (1 mg mL−1), stored in quartz cuvettes and irradiated with a pulsed laser at 808 nm under continuous stirring at laser output power varying between 0.4 W and 2.6 W. Temperature increment over 5 min was recorded with a 10 s time-step by means of a thermocouple in direct contact with the dispersion and with a thermal camera. Temperature increase up to 58 °C was recorded with the maximum laser power, whereas 1.1 W laser output resulted in the minimum power allowing the HT temperature threshold to be exceeded in about 250 s (Fig. S4, ESI†). Furthermore, the thermal images confirm that water-dispersed tNAs are uniformly heated up to 42 °C after 5 minutes of irradiation (Fig. 2A). It is worth noticing (Fig. 2B) that: (i) laser irradiation of both pure water or water solutions of nano-architectures devoid of metals (metal-free NAs, mfNAs) under the same experimental conditions results in a negligible temperature increase, and (ii) irradiation of standard NAs produced a less efficient PT conversion with respect to tNAs, likely due to optical excitation on the tail of LSPR. Taken together, these findings confirm that PT conversion is due to a plasmonic effect rather than to the presence of silica and polymers. An interesting feature of tNAs is related to their potential employment for repeated photothermal cycles avoiding damage or re-shaping. Indeed, we assessed PT transduction efficacy at fixed laser output power 1.1 W, demonstrating that light-to-heat conversion is not affected after four cycles of 5 min each (Fig. 2C). Notably, TEM investigations revealed that, although intense laser irradiation induced the rupture of some nanocapsules, the overall morphology of tNAs is preserved (Fig. S5, ESI†). In particular, after four PT cycles no reshaping or sintering effect is produced on gold USNPs, which retain their optical behavior suitable for PT as well as their ideal ultrasmall size for renal clearance.25
PT effect of tNAs was also evaluated on 2D and 3D models of PDAC. The choice of the MiaPaCa-2 cell line was determined by its particular aggressiveness and tendency to form metastases among PDAC models, and owing to our long-standing experience with this line, whose behavior has been fully assessed in our previous works.23,28,29 In order to follow the cellular uptake of NAs and tNAs, AlexaFluor 647 dye was included into the nanocapsules, and imaging was performed through confocal fluorescence microscopy.23 Fig. S6 (ESI†) shows the uptake of both NAs and tNAs in 2D cultured MIA PaCa-2 cells after 2 h incubation. As expected, there is no appreciable difference between the cellular internalization of the two nano-architectures since their overall external morphology is preserved, confirming the robustness of the synthetic protocol. Notably, viability assays performed after 72 hours from the incubation, showed that the presence of DMSB in the tNAs does not induce a cytotoxic effect (Fig. S7, ESI†).
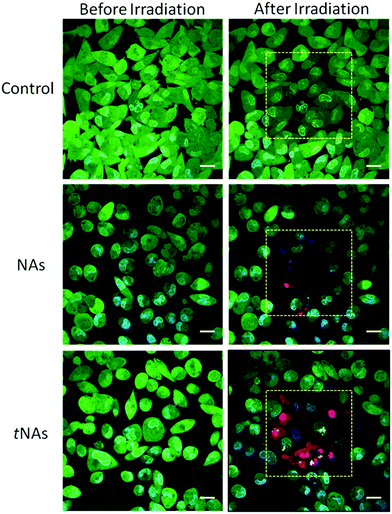
For studying PT treatment on 2D cultures, cells were incubated with NAs and tNAs (30 μg) for 2 hours and, then, laser irradiation was performed through confocal microscopy; a region of interest of 512 × 512 pixels (57 × 57 μm) was scanned with a pixel dwell time of 40 μs per pixel for 225 s employing a laser output power of 17 mW. This value was chosen as the maximum power not-inducing cellular death in the control samples (untreated cells) under the same irradiation timeframe. HT efficacy has been qualitatively evaluated through calcein-AM/propidium iodide (PI) cell staining. After the treatment, no sign of cellular death was found in the control (Fig. 3, top), yet there was a slight dimming of the green fluorescence likely due to calcein bleaching. On the other hand, HT induced by laser excitation of tNAs-treated cells caused substantial cellular death (Fig. 3, bottom). PT generated by NAs produced a less prominent cytotoxic effect (Fig. 3, center) in agreement with the PT efficiency assessments in water, as NAs cause faint heating not sufficient to result in HT or to induce damage to cells.
Then, the cytotoxic effect of tNAs-induced HT was assessed on 3D models of PDAC. Findings obtained on 3D models have a more significant potentiality of translation with respect to both standard 2D cell cultures and subcutaneous xenograft in vivo models.30 Furthermore, the development of customized 3D neoplasm models is in agreement with the 3R's concept, avoiding the sacrifice of animal models unless strictly necessary. Finally, 3D models enable potential investigations over the physiology of neoplasms in physiological conditions, including cell–cell and cell–extracellular matrix (ECM) interactions.31,32 For example, light scattering from 3D multicellular spheroids has been previously investigated, reporting no significant dependence on the presence of cell-to-cell junctions or ECM, while scattered light's angular distribution is strictly dependent on the refractive indexes of different cellular compartments, exactly as in 2D models.33 The 3D MIA PaCa-2 cell cultures were prepared through the hanging method, as fully described elsewhere.29 Briefly, after 24 hours of drop suspension, the cell aggregates were transferred and maintained in an incubator with an orbital shaker for up to 1 month. The diameter of the obtained spheroids can range from 250 μm to about 1 mm. Herein, in order to both improve the significance of the comparison with samples and to approach models with a micrometastasis resemblance (whose diameter is usually in the range of 0.2–2 mm), we have selected spheroids with a starting diameter of 350–450 μm (Fig. S8, ESI†).34
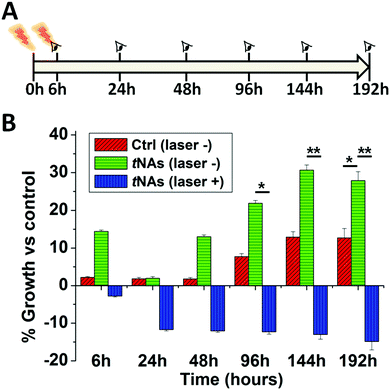
The best equilibrium between particle incubation and 3D model internalization was comprehensively investigated elsewhere for both bare and targeted NAs.29 As expected, the internalization behavior of tNAs (Fig. S9, ESI†) resulted in agreement with NAs. HT efficacy was evaluated as spheroid growth inhibition over 192 hours after PT treatments. Spheroids were irradiated at 808 nm with an irradiance of 80 mW cm−2 for 10 minutes after 2 hours incubation with tNAs. The treatment was repeated after 6 hours with the same exposure conditions, and spheroid growth was monitored over 8 days (Fig. 4A).
Double irradiation was performed in order to damage cells both on the membranes and in the cytosol. Remarkably, spheroids were irradiated over their entire volume as, in our set-up, a semiconductor CW laser diode was employed in order to enhance the perspective of clinical translation. The irradiations produced a significant inhibition of the growth of the models treated by tNAs (Fig. 4B). On the other hand, tNAs themselves did not show any evidence of induced cytotoxicity. Indeed, models incubated with tNAs, yet not irradiated, demonstrated a regular growth similar to the not-irradiated control. Even if the PT treatment is not sufficient to induce complete spheroid death, the damage induced by laser irradiation has a major effect on cell viability (Fig. S10, ESI†) after 24 hours and results in growth inhibition that extends over days.
Conclusions
Overall, tNAs are the first reported NIR-absorbing plasmonic ultrasmall-in-nano platforms that jointly combine: (i) PT conversion efficacy suitable for HT, (ii) the possibility of multiple PT series and (iii) renal excretion of the building blocks after the therapeutic action. It is worth mentioning that tNAs sustain all the features of standard NAs, including the possibility of encapsulating drugs in their hollow cavity for HT-assisted chemotherapy. Their therapeutic effect has been assessed in valuable 3D models of human pancreatic adenocarcinoma. Moreover, tissue heating arising from NIR laser exposure could be effectively exploited for photoacoustic-guided HT, in order to employ a single nanostructure for simultaneous detection and treatment of diseases.Materials and methods
AlexaFluor-647 was purchased from Invitrogen. All the other chemicals were provided by Sigma-Aldrich. Every reactant was used as it was without further purification.Synthesis of passion fruit-like nano-architectures (NAs)
The following protocol is standardized for the production of 1.5 mg NAs in about 4 h. The protocol can be scaled-up to 20 mg.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm for 3 minutes), suspended in 2 mL of milliQ water and sonicated for a maximum of 4 minutes.
400 rpm for 3 minutes), suspended in 2 mL of milliQ water and sonicated for a maximum of 4 minutes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm, (ii) remove the colorless supernatant, and (iii) add the solvent of interest. The solubility of NAs in water, buffers, and physiological fluids is tested for up to 60 mg mL−1.
400 rpm, (ii) remove the colorless supernatant, and (iii) add the solvent of interest. The solubility of NAs in water, buffers, and physiological fluids is tested for up to 60 mg mL−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of 70% glycerol and FBS. Imaging was performed on an Olympus FV1000 inverted confocal laser scanning microscope equipped with a thermostat chamber set at 37 °C and 5% CO2. The lasers for excitation were 405, 488, and 633 nm. Imaging was done 24 h after incubation. All images were analyzed using Fiji-ImageJ software version 1.51 s.
1 solution of 70% glycerol and FBS. Imaging was performed on an Olympus FV1000 inverted confocal laser scanning microscope equipped with a thermostat chamber set at 37 °C and 5% CO2. The lasers for excitation were 405, 488, and 633 nm. Imaging was done 24 h after incubation. All images were analyzed using Fiji-ImageJ software version 1.51 s.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The research leading to these results has received funding from AIRC under MFAG 2017 – ID 19852 project – P. I. Voliani Valerio. We thank Ms Gina Greco for her support with thermal camera measurements.References
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Photochem. Photobiol., 2006, 82, 412 CrossRef CAS PubMed.
- M. F. Tsai, S. H. G. Chang, F. Y. Cheng, V. Shanmugam, Y. S. Cheng, C. H. Su and C. S. Yeh, ACS Nano, 2013, 7, 5330–5342 CrossRef CAS PubMed.
- Y. Liu, X. Zhi, M. Yang, J. Zhang, L. Lin, X. Zhao, W. Hou, C. Zhang, Q. Zhang, F. Pan, G. Alfranca, Y. Yang, J. M. de la Fuente, J. Ni and D. Cui, Theranostics, 2017, 7, 1650–1662 CrossRef CAS PubMed.
- I. G. Theodorou, Z. A. R. Jawad, Q. Jiang, E. O. Aboagye, A. E. Porter, M. P. Ryan and F. Xie, Chem. Mater., 2017, 29, 6916–6926 CrossRef CAS.
- A. Li Volsi, C. Scialabba, V. Vetri, G. Cavallaro, M. Licciardi and G. Giammona, ACS Appl. Mater. Interfaces, 2017, 9, 14453–14469 CrossRef CAS PubMed.
- R. S. Riley and E. S. Day, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 9, e1449 CrossRef PubMed.
- A. C. Anselmo and S. Mitragotri, AAPS J., 2015, 17, 1041–1054 CrossRef CAS PubMed.
- F. Chen and W. Cai, Nanomedicine, 2015, 10, 1–3 CrossRef CAS PubMed.
- T. Sun, Y. S. Zhang, B. Pang, D. C. Hyun, M. Yang and Y. Xia, Angew. Chem., Int. Ed., 2014, 53, 12320–12364 CAS.
- Y. Vlamidis and V. Voliani, Front. Bioeng. Biotechnol., 2018, 6, 143 CrossRef PubMed.
- E. Lim, T. Kim, S. Paik, S. Haam, Y. Huh and K. Lee, Chem. Rev., 2015, 115, 327–394 CrossRef CAS PubMed.
- K. Jiang, D. A. Smith and A. Pinchuk, J. Phys. Chem. C, 2013, 117, 27073–27080 CrossRef CAS.
- F. Zhao, X. Li, J. Li, Y. Dou, L. Wang, M. Wu, Y. Liu, J. Chang and X. Zhang, J. Mater. Chem. B, 2017, 5, 2145–2151 RSC.
- M. Gordel, J. Olesiak-Banska, K. Matczyszyn, C. Nogues, M. Buckle and M. Samoc, Phys. Chem. Chem. Phys., 2014, 16, 71–78 RSC.
- G. González-Rubio, A. Guerrero-Martínez and L. M. Liz-Marzán, Acc. Chem. Res., 2016, 49, 678–686 CrossRef PubMed.
- N. Katchinskiy, R. Godbout, A. Hatef and A. Y. Elezzabi, Adv. Ther., 2018, 1, 1800009 CrossRef.
- Y.-S. Chen, W. Frey, S. Kim, K. Homan, P. Kruizinga, K. Sokolov and S. Emelianov, Opt. Express, 2010, 18, 8867 CrossRef CAS PubMed.
- D. Cassano, S. Pocoví-Martínez and V. Voliani, Bioconjugate Chem., 2018, 29, 4–16 CrossRef CAS PubMed.
- J. G. Croissant, Y. Fatieiev and N. M. Khashab, Adv. Mater., 2017, 29(9), 1604634 CrossRef PubMed.
- D. Cassano, D. Rota Martir, G. Signore, V. Piazza and V. Voliani, Chem. Commun., 2015, 51, 9939–9941 RSC.
- C. Avigo, D. Cassano, C. Kusmic, V. Voliani and L. Menichetti, J. Phys. Chem. C, 2017, 121, 6955–6961 CrossRef CAS.
- P. Armanetti, S. Pocoví-Martínez, A. Flori, C. Avigo, D. Cassano, L. Menichetti and V. Voliani, Nanomedicine, 2018, 14, 1787–1795 CrossRef CAS PubMed.
- D. Cassano, M. Santi, V. Cappello, S. Luin, G. Signore and V. Voliani, Part. Part. Syst. Charact., 2016, 818–824 CrossRef CAS.
- M. D’Amora, D. Cassano, S. Pocoví-Martínez, S. Giordani and V. Voliani, Nanotoxicology, 2018, 914–922 CrossRef PubMed.
- D. Cassano, M. Summa, S. Pocoví-Martínez, A.-K. Mapanao, T. Catelani, R. Bertorelli and V. Voliani, Part. Part. Syst. Charact., 2018, 1800464 Search PubMed.
- S. Pocoví-Martínez, D. Cassano and V. Voliani, ACS Appl. Nano Mater., 2018, 1, 1836–1840 CrossRef.
- D. Cassano, J. David, S. Luin and V. Voliani, Sci. Rep., 2017, 7, 43795 CrossRef CAS PubMed.
- M. H. Katz, S. Takimoto, D. Spivack, A. R. Moossa, R. M. Hoffman and M. Bouvet, Clin. Exp. Metastasis, 2004, 21, 7–12 CrossRef CAS PubMed.
- A. K. Mapanao, M. Santi, P. Faraci, V. Cappello, D. Cassano and V. Voliani, ACS Omega, 2018, 3, 11796–11801 CrossRef CAS PubMed.
- S. J. Jackson and G. J. Thomas, Dis. Models Mech., 2017, 10, 939–942 CrossRef CAS PubMed.
- S. Nath and G. R. Devi, Pharmacol. Ther., 2016, 163, 94–108 CrossRef CAS PubMed.
- H. Lu and M. H. Stenzel, Small, 2018, 14, 1702858 CrossRef PubMed.
- J. R. Mourant, T. M. Johnson, V. Doddi and J. P. Freyer, J. Biomed. Opt., 2002, 7, 93 CrossRef CAS PubMed.
- P. Hermanek, R. V. P. Hutter, L. H. Sobin and C. Wittekind, Cancer, 1999, 86, 2668–2673 CrossRef CAS PubMed.
- R. Foty, J. Visualized Exp., 2011 DOI:10.3791/2720.
- M. Ménard, F. Meyer, K. Parkhomenko, C. Leuvrey, G. Francius, S. Bégin-Colin and D. Mertz, Biochim. Biophys. Acta, Gen. Subj., 2019, 1863, 332–341 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9mh00096h |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |