A two-photon AIEgen for simultaneous dual-color imaging of atherosclerotic plaques†
Abstract
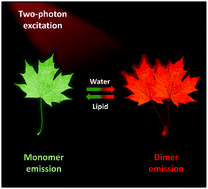
High-resolution imaging of lipids within the artery is of great value to obtain fundamental knowledge about their roles in atherosclerosis, but ideal probes are still lacking. In this study, we report a smart probe, namely, IND with aggregation-induced emission property for two-photon visualization of lipids within cell lines and arteries. Taking advantage of high lipid specificity, deep tissue penetrability, and excellent two-photon imaging performance, IND is capable of high-resolution imaging of lipids in cultured cells and in situ mapping of three-dimensional lipid distributions in mouse atherosclerotic plaques without tedious histological preparations. Importantly, we find that IND exhibits distinct emissive properties in the molecular monomer and dimer states; also, we uncover its remarkable applications for single-excitation dual-color imaging of lipid/water in the tissue microenvironment to reveal the ultra-structure of atherosclerotic plaques. The findings described herein provide a simple tool with minimal disruptive procedures for studying atherosclerosis at the tissue level and a powerful strategy for the development of imaging-based diagnostic techniques with color switching capability.
- This article is part of the themed collections: Horizons Community Board Collection: Biosensors and Aggregation-Induced Emission with Bin Liu and Ben Zhong Tang


 Please wait while we load your content...
Please wait while we load your content...