The global antibiotic research and development partnership (GARDP): researching and developing new antibiotics to meet global public health needs
Laura J. V.
Piddock

Global Antibiotic Research and Development Partnership, 15 Chemin Louis-Dunant, 1202 Geneva, Switzerland. E-mail: lpiddock@gardp.org; Web: www.gardp.org
GARDP is a not-for-profit research and development (R&D) organization developing and delivering new or improved antibiotics. Through partnerships, collaborations, and coordination, GARDP works with stakeholders from across the world to develop new treatments in a way that is sustainable and ensures access to all. Launched in May 2016 by the World Health Organization (WHO) and the Drugs for Neglected Diseases Initiative (DNDi), GARDP is an important element of the WHO's global action plan on antimicrobial resistance that calls for new public–private partnerships to encourage research and development of new antimicrobial agents and diagnostics. When defining which antibiotics to develop, GARDP considers the priority pathogens identified by the WHO for which there is a need for new drugs, as well as the needs and gaps in the treatment of infectious diseases in key populations. In this way, GARDP's efforts focus on global public health priorities and indications less likely to be developed by others.
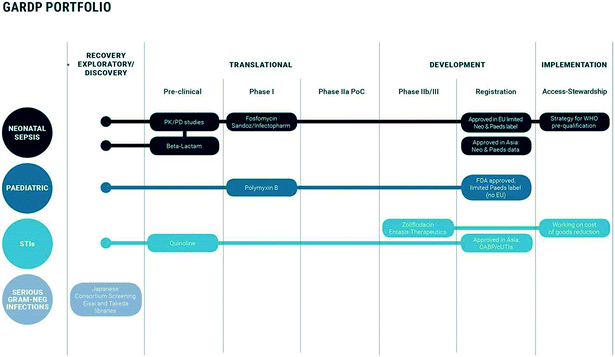
Currently, there are three clinical development programmes focused on developing antibiotics for sexually transmitted infections, neonatal sepsis, and paediatric infections. There is also a transversal antimicrobial memory recovery and a discovery and exploratory R&D programme. GARDP’s model is ‘bench to bedside’ – a drug developer with the ability to enter any point along the drug development pipeline, from discovery and early exploratory research to preclinical and clinical development and ultimately access to patients. Addressing AMR is a global public-health priority requiring multiple approaches. One commonly agreed approach is to ensure good antimicrobial stewardship of new antibiotics, i.e. use appropriately and only to treat bacterial infections. In its clinical programmes, GARDP strives to embed good antimicrobial stewardship whilst ensuring access to its medicines to all those who need them.
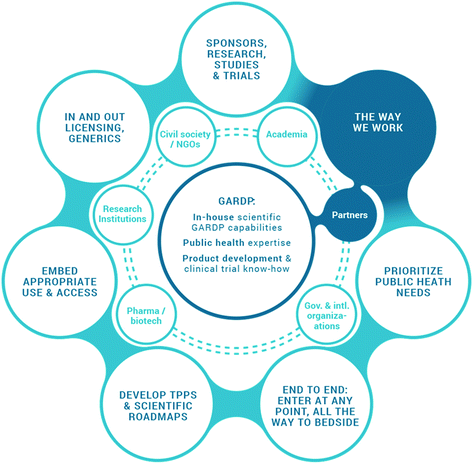
GARDP aims to develop four new treatments by 2023; these will be effective for infections caused by bacteria on the WHO priority pathogens list for R&D of new antibiotics (priorities 1 and 2: critical and high priority pathogens) (Table 1).1 This will be achieved either by improving existing drugs or by developing new chemical entities. To achieve this, GARDP has entered into multiple partnerships with industry, research institutions and academia, governments, and other not-for-profit organisations (Fig. 1). These partnerships are built around sharing costs, risks, and benefits of antibiotic R&D programmes and are based on a collaboration of expertise, capacity and capability to overcome the substantial challenge of providing treatment options where they are currently limited. Strategic R&D partnerships are based on a mutual understanding of where each organisation can align and synergise based on respective capabilities within the framework of a memorandum of understanding, which can be translated into a partnership agreement covering various components of R&D projects. Importantly, GARDP takes a portfolio approach when building global partnerships and making long-term commitments to projects and partners (Fig. 2). There are considerable development risks when conducting and sponsoring complex R&D programs with multiple partners, even when partners have expertise in CMC, regulatory, manufacturing and supply.
| WHO priority 1: CRITICAL | GARDP programme |
|---|---|
| Carbapenem-resistant Acinetobacter baumannii | Neonatal sepsis, paediatrics and AMREP |
| Carbapenem-resistant Pseudomonas aeruginosa | None |
| Carbapenem-resistant 3rd generation cephalosporin-resistant Enterobacterialesa | Neonatal sepsis, paediatrics and AMREP |
| Priority 2: HIGH | |
|---|---|
| a Enterobacteriales include: Klebsiella pneumoniae, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp, and Morganella spp. | |
| Vancomycin-resistant Enterococcus faecium | None |
| Methicillin-resistant and vancomycin intermediate and resistant Staphylococcus aureus | None |
| Staphylococcus aureus | None |
| Clarithromycin-resistant Helicobacter pylori | None |
| Fluoroquinolone-resistant Campylobacter spp. | None |
| Fluoroquinolone-resistant Salmonella spp. | None |
| 3rd generation cephalosporin-resistant and fluoroquinolone-resistant Neisseria gonorrhoeae | Sexually transmitted infections |
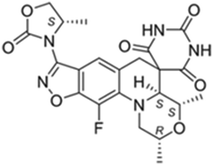
The first clinical development programme initiated by GARDP focuses on developing antibiotics for sexually transmitted infections. This programme aims to deliver one treatment that will be effective against drug-sensitive and drug-resistant forms of gonorrhoea and ideally be efficacious against infections of the genital tract as well as extra-genital infections. This treatment will need to be integrated into the current treatment paradigm, that often entails empiric treatment in the absence of adequate diagnosis. The first drug being developed in partnership with an SME, Entasis Therapeutics, is zoliflodacin, a novel inhibitor of bacterial DNA gyrase B2 (Fig. 3). Six successful clinical trials (including three NIAID-sponsored enabling phase 2 trials and a GARDP-sponsored food effect trial, https://www.clinicaltrials.gov/ct2/results?cond=&term=zoliflodacin&cntry=&state=&city=&dist=)3,4 have paved the way for a GARDP-sponsored phase 3 pivotal clinical trial due to start in 2019.
The neonatal sepsis clinical programme aims to deliver a treatment that can be given empirically to babies with infections presumed to be caused by drug-resistant Gram-negative bacteria. This means that such a drug would only need to be used in settings where there is significant resistance to the WHO current standard of care for neonatal sepsis, ampicillin and gentamicin. This strategy aims to minimise the emergence of drug resistance to any new drugs developed by GARDP and its partners and could offer a life-saving alternative in areas with a high incidence of multidrug resistant infections. To inform the choice of drugs and design of future phase 3 clinical trials in babies, GARDP is currently carrying out a global observational study in eleven different countries to understand the current diagnosis and treatment of sepsis in neonates (Table 2). As part of this study, where possible, bacterial cultures are obtained, and these will be whole genome sequenced. This data will help to inform the community on prevalence of bacterial species and drug resistances. A phase 1 pharmacokinetic and safety study of fosfomycin in neonates completed enrolment in February 2019.
| Country | Hospital |
|---|---|
| GARDP is the sponsor of the clinical trial; St. George's Hospital, University of London, UK is the clinical coordinator; the penta Foundation is administrating the approvals and contracts; the University of Antwerp is providing microbiology support. | |
| Bangladesh | Dhaka Shishu Hospital, Dhaka |
| Brazil | Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto |
| Brazil | FCM da Santa Casa de São Paulo |
| China | Shenzhen Children's Hospital, Shenzhen |
| China | Beijing Children's Hospital, Beijing |
| China | Beijing Obstetrics and Gynecology Hospital, Beijing |
| Greece | Hippokration Hospital, Thessaloniki |
| India | Lady Hardinge Medical Centre (LHMA), Delhi |
| India | Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry |
| India | King Edward Memorial (KEM) Hospital and Seth Gordhandas Sunderdas Medical College, Mumbai |
| Italy | Bambino Gesù Hospital, Rome |
| Kenya | KEMRI/Wellcome Trust Research Programme, Kilifi |
| South Africa | Tygerberg Children's Hospital, Cape Town |
| South Africa | Chris Hani Baragwanath Academic Hospital, Johannesburg |
| South Africa | Charlotte Maxeke Academic Hospital, Johannesburg |
| Thailand | Chiang Rai Prachanukroh Hospital (PHPT), Chiang Rai |
| Thailand | Queen Sirikit National Institute of Child Health, Bangkok |
| Uganda | Mulago Hospital, Kampala |
| Vietnam | Vietnam National Children's Hospital, Hanoi |
The development of antibiotics to treat infections in children often lags behind that for new drugs for adults. Consequently, the formulation and dosing regimen of antibiotics for children is not always optimal. The GARDP paediatric programme aims to extend the use, improve and optimise formulations of existing antibiotics and accelerate the development of new antibiotics for use in childhood infections. Sandoz has partnered with GARDP to help achieve these aims. Specifically, this programme aims to develop a new treatment where the identity of the causative bacterium is known and confirmed as multidrug resistant including resistance to carbapenems. The first project will evaluate polymyxin B.
The antimicrobial memory recovery and exploratory programme (AMREP) is comprised of three components. The first is memory recovery and asset evaluation that seeks to identify undeveloped or underused old antibiotics, clinically efficacious combinations of antibiotics, as well as new chemical entities that can be developed into new treatments for GARDP's clinical programmes. Currently, two recovered drugs are being evaluated; one with the University of Liverpool, UK, the other by NIAID, USA. A systematic review of combinations of antibiotics is also underway in collaboration with the University of Verona. Secondly, GARDP has established an education, training and outreach programme on antimicrobial R&D called REVIVE (https://revive.gardp.org/https://revive.gardp.org/webinars/). REVIVE has developed a variety of resources and activities to connect established researchers and those entering the field of antimicrobial drug discovery, research and development, including early career researchers. Starting in September 2017, GARDP partnered with CARB-X to organise and host symposia at international conferences, and with the Pew Charitable Trusts to organise networking events. More recently, GARDP is collaborating with CARB-X, the REPAIR Impact Fund, the Wellcome Trust and the EU JPI-AMR to organise educational events at relevant international conferences. REVIVE also organises and hosts monthly webinars (https://revive.gardp.org/webinars/). The regular blogs include a personal viewpoint by Baron (Jim) O'Neill of Gatley on economic models for antibacterial R&D including the alternative approach offered by GARDP; other topics include ‘Recent approvals – do they make a difference?’ by Ursula Theuretzbacher, ‘Why we need to prioritize developing antibiotics for children’ by the GARDP Executive Director, Manica Balasegaram, ‘Time to pull out the stops in the fight against superbugs’ by Kevin Outterson (https://revive.gardp.org/blog/). GARDP is actively developing resources for the community, including links to key conferences in the field, and relevant databases. Currently under development, and due to be launched in 2019, is an antimicrobial R&D encyclopaedia. This will include definitions of commonly used terms, videos and links to resources describing methods (e.g., the international standardised methods for determining drug resistance).
The third component of AMREP is the discovery and exploratory programme. Partnerships have been established with the two Japanese pharmaceutical companies (Eisai and Takeda) and the Institut Pasteur Korea. In 2019, there will be further additional screening partnerships (with two academic institutions and a not-for-profit organisation). These collaborations with GARDP allow the screening of proprietary libraries of natural products and synthetic compounds for activity against drug-resistant Gram-negative bacteria. To identify those with whom GARDP may want to partner to help develop their assets, GARDP will do a horizon scanning project to identify academic groups and SMEs who are carrying out discovery and early exploratory research on new chemical entities and/or new targets in Gram-negative bacteria.
The overall objective of GARDP is to deliver four new and/or improved treatments by 2023 for infections and populations at most need and to build a robust pipeline for the long term. The rapid development of GARDP into an independent Swiss Foundation, support from numerous governments currently totalling €66 million, and its new partnerships, demonstrates the support for a not-for-profit model and offers optimism for the R&D of new antibiotics.
References
- Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics, 2017, https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 Search PubMed.
- S. Jacobsson, D. Golparian, R. A. Alm, M. Huband, J. Mueller, J. S. Jensen, M. Ohnishi and M. Unemo, High in vitro activity of the novel spiropyrimidinetrione AZD0914, a DNA gyrase inhibitor, against multidrug-resistant Neisseria gonorrhoeae isolates suggests a new effective option for oral treatment of gonorrhea, Antimicrob. Agents Chemother., 2014, 58(9), 5585–5588 CrossRef PubMed.
- S. N. Taylor, J. Marrazzo, B. E. Batteiger, E. W. Hook 3rd, A. C. Seña, J. Long, M. R. Wierzbicki, H. Kwak, S. M. Johnson, K. Lawrence and J. Mueller, Single-Dose Zoliflodacin (ETX0914) for Treatment of Urogenital Gonorrhea, N. Engl. J. Med., 2018, 379(19), 1835–1845 CrossRef CAS PubMed.
- J. O'Donnell, K. Lawrence, K. Vishwanathan, V. Hosagrahara and J. P. Mueller, Single-Dose Pharmacokinetics, Excretion, and Metabolism of Zoliflodacin, a Novel Spiropyrimidinetrione Antibiotic, in Healthy Volunteers, Antimicrob. Agents Chemother., 2018, 63, 1808–1818 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |