 Open Access Article
Open Access ArticleLipidomic analysis as a tool for identifying susceptibility to various skin diseases
Valeriy V.
Smirnov
 ab,
Evgenii A.
Egorenkov
b,
Tatiana N.
Myasnikova
b,
Alexey E.
Petukhov
a,
Vladimir I.
Gegechkori
ab,
Evgenii A.
Egorenkov
b,
Tatiana N.
Myasnikova
b,
Alexey E.
Petukhov
a,
Vladimir I.
Gegechkori
 a,
Anna M.
Sukhanova
a,
Anna M.
Sukhanova
 a and
Galina V.
Ramenskaya
a and
Galina V.
Ramenskaya
 *a
*a
aI.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), 119991 Moscow, Russia. E-mail: ramenskaia@mail.ru
bNRC Institute of Immunology FMBA of Russia, 115478 Moscow, Russia
First published on 12th September 2019
Abstract
This review is about the significance of the use of lipidomic analysis for identifying susceptibility to skin diseases. Exactly this article describes the use of lipidomic analysis in different studies to detect abnormalities in the lipid composition of the skin to diagnose and prevent various dermatological diseases.
Human skin is the largest organ in size and weight, and it performs three main functions: regulation, sensation and protection. According to its structure, the skin consists of several layers: epidermis and dermis, and under the dermis lies the hypodermis or subcutaneous fatty tissue. Many different factors affect the lipid composition of the skin. The main ones are age, gender, ethnicity and season. Also, changes in the amount of lipids can be caused by various pathological processes, both in the skin and in the whole system: hereditary ichthyosis, atopic dermatitis, Netherton syndrome, and others.1 Lipidomics is the determination of the qualitative and quantitative composition of lipids in various organ systems and tissues. According to the results in this field, it is possible to make a complete lipid profile both in cells and in tissues. Also with the inclusion of data on the composition of proteins, sugars and nucleic acids in tissues, the difference in structures between healthy and affected tissues can be described with the subsequent use of the obtained information for the diagnosis and development of new treatment approaches. Such studies also include an assessment of the interaction of the studied substances with other proteins and lipids, as well as the structure, function, and dynamic changes of cellular lipids in normal and various pathologies.
Lipidomic analysis of normal skin
For the successful lipidomic analysis of the skin, it is necessary to develop an accurate and reproducible sampling technique, since the composition of the skin also depends on its location. First, the method must extract a certain amount of biomaterial with the same accuracy in order to ensure the reliability of the obtained data on the skin lipid composition. This is especially important when studying changes in lipid composition in the event of pathology, since physiological processes vary. The sampling procedure should not in any way affect the initial composition of the tissue, and should neither introduce nor remove lipids and other substances from the sample.2Currently, there are several methods of sampling the skin for lipidomic analysis. For example, the method of biopsy scraping allows withdrawal of a sufficient amount of sample with minimal risk of contamination, but it is not easily possible to determine the exact number and depth of the sample being extracted. The so-called “cup method”, which can be described as in situ extraction, provides complete material extraction without contamination, but does not have high reproducibility and is relatively invasive.3 One of the most promising methods is tape-stripping, which allows control of the position, quantity and depth of the biomaterial being extracted. However, it should be noted that the subsequent extraction of lipids with organic solvents may lead to the appearance of foreign substances in the sample that may interfere with the subsequent analysis.
A study examined how the lipid composition of the skin varies depending on the depth of the layer.4 Samples for that study were taken from the left forearms of both male and female individuals using tape-stripping. The layers were extracted successively from the same point, and a total of 20 layers were removed, after which the lipid composition of each was examined. As a result, it was found that the total amount of lipids is reduced by 85% in the 5th layer in females and 87% in the 7th layer in males, after which it reaches a plateau. This reduction can be caused by the larger mass of the first layer samples, by the higher proportion of lipids to the rest of the substances in the sample, or by both.
The upper layers of the skin, having a greater mass, also contain a greater amount of lipids. It was shown that the amount of sebum lipids on the surface of the skin is 10 times more than the amount of lipids in the stratum corneum (SC).
In both sexes, a marked decline in the relative amount of triacylglycerols (TAGs) and diacylglycerols (DAGs) was observed, jointly accounting for half of the known sebum lipids on average, following the decrease in the total lipid amount. This supports the notion of sebum lipid penetration into the SC down to the 5–7th layers, where TAG and DAG levels reach their plateaus.
As the layers deepened, the amount of cholesterol and ceramides increased significantly. Such a concentration gradient confirms that ceramide synthesis occurs in the deep layers of the SC, although they are not components of sebum. Similarly, the proportion of cholesterol in the total amount of SC lipids reaches one third, although in sebum it is represented in a much smaller amount, thus making up a small part of lipids on the skin surface.
As a rule, prominent lipid profiles are rarely observed, and the lipid composition of skin cells is approximately the same, although it differs by sex. The main contribution to the differences in lipid composition is the components of sebaceous lipids, which are the major factors responsible for sample separation and clustering.
A large inter-individual variability of the amount of skin lipids was detected in samples taken from the volar forearm compared to the intra-individual variability of the amount of lipids taken from 14 different sites from one male or female. In addition, there is a decrease in the total and relative amount of sebaceous lipids depending on the age of the subject, especially among females, as mentioned in several studies (Fig. 1).5,6
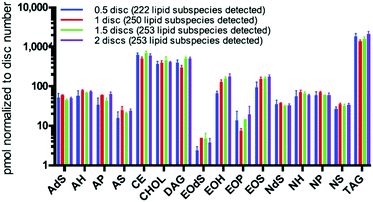 | ||
| Fig. 1 Comparison of lipid class amounts normalized to number of stripping discs extracted. The skin was extracted from the forearm with the tape-stripping skin collection method. AdS, AH, AP, AS, EOH, EOP, EOS, NDS, NH, NP, and NS are ceramide subtypes; CHOL – cholesterol.4 | ||
Lipidomic analysis and inflammatory skin disease progression
Lipidomic analysis is starting to be widely used to study the dynamic changes in the skin composition in the event of local or systemic inflammatory processes. Published studies proved the effect of changing the lipid composition of the skin on the impairment of the protective function of the skin, which may be the main cause of a number of inflammatory diseases.Atopic dermatitis is an inflammatory skin disease, the cause of which is mainly the impairment of the barrier functions of the epidermis. This condition is characterized by an increased transepidermal water loss (TEWL). The impaired barrier function of skin permeability in AD is often associated with changes in lipids, especially ceramides (CERs).7,8
Classes of CERs have been studied, the content of which is significantly reduced in the skin with AD compared to healthy intact skin – CER[NP], CER[EOH], CER[NH], CER[EOS] and CER[EOP]. In addition to reducing the overall level of ceramides, there was a constant decrease in the quantitative content of CERs with a short chain length or an increased content of CERs with a long chain length.9 By measuring the TEWL, the dependence of the decrease in the barrier function of the permeability of the SC on the increase in the quantitative content of the CERs with a modified chain length was determined. Moreover, it was demonstrated that the severity of the disease course depends on factors such as the degree of change in the quantitative composition of CERs and disorders in the organization of the lipid chain, primarily impairing the skin barrier function and also exhibiting an effect on the activity of natural moisturizing factors in the skin, derived from filaggrin.10
Netherton syndrome (NTS) is a skin disease caused by a mutation in the gene encoding a serine protease inhibitor, the lympho-epithelial Kazal-type-5 inhibitor (LEKTI). As in the case of atopic dermatitis, the disease develops due to the impairment of the barrier function of the skin due to the increased activity of peptidases that affect the level of kallikrein – a serine protease catalyzing the formation of kinin from kininogens. The clinical picture of the disease shows chronic inflammation of the skin typical of nonbullous ichthyosiform erythroderma, as well as multiple defects in the structure of the spinous hair (the presence of “bamboo hair”). Lipidomic analysis of the skin of patients with Netherton syndrome showed a decrease in the length of free fatty acid (FFA) chains, as well as an increase in the level of monounsaturated fatty acids (MUFA) in the SC compared with healthy people. A decrease in the skin barrier function was also associated with an increase in the quantitative content of short-chain CERs and the presence of unsaturated CERs of various subclasses with different chain lengths. In addition to reducing the level of the CERs, patients with NTS also have a reduced level of acyl-CERs, which are the precursors and transport forms of CERs, which also adversely affect the functional state of the skin.
Having studied the lipid profiles of the quantitative content of various subclasses of FFAs and CERs in patients with NTS, it was determined that the composition of these lipids in the skin corresponds to the altered activity of enzymes responsible for the synthesis of lipids in the SC. Thus, the results of lipidomic analysis can help in the selection of stabilization strategies for this syndrome in order to increase the barrier function of the skin and replenish the required level of lipids in the SC.11
Psoriasis is a skin disease associated with impaired epidermal homeostasis and a decreased skin barrier function due to autoimmune processes resulting in the formation of red, excessively dry papules and plaques above the skin surface. By means of lipidomic analysis, it was determined that changes in the quantitative content and distribution of CERs in the SC affect the impairment of the functional state of the skin. There were significant differences in the quantitative composition of certain subclasses of CERs in patients with psoriasis and healthy volunteers – CER [ADS], [NP], CER [NH] and CER [AP]. There was a significant decrease in the proportion of CERs with long-chain fatty acids in the skin of patients with psoriasis. Moreover, it was determined that in patients with psoriasis not only the composition of the skin CERs was altered, but also the level of sphingoid bases – substances that have an inhibitory effect on many cellular functions, including the processes of proliferation, differentiation and programmed cell death, activates other kinases and has a regulatory influence on the whole spectrum of enzymes involved in extracellular signaling. The levels of sphingosine and sphinganine in the psoriatic epidermis were increased, and the severity of the clinical course of psoriasis is directly related to the increased activity of ceramidase.
Thus, these studies have proven the effect of changes in the lipid composition of SC on the severity of psoriasis and the severity of changes in the barrier function of the skin.
Due to the large variation in changes in the qualitative and quantitative composition of the skin in psoriasis, in order to further understand the role of SC lipids in maintaining the skin barrier function in patients, a more detailed lipidomic analysis should be performed with an increase in samples from patients with varying degrees of disease.12,13
Acne is an inflammatory skin disease caused by changes in pilosebaceous structures (composed of hair follicles and sebaceous glands). To date, it has been established that the development of acne depends primarily on changes in surface skin lipids (SSL), hormone levels and bacterial infection. Additional factors for the development of acne include the lipid composition of the blood, presence of bacteria in skin, diet and degree of obesity. It is also known that the occurrence of acne is closely related to lipid hypersecretion induced by androgen hormones, causing changes in skin lipid composition compared to normal skin.14 It was found that sapienic acid, a major fatty acid in human sebum, inhibits the growth of acne related bacteria. Lack of sapienic acid can be the cause of acne.15 It is believed that the lipid composition of the SSL is fundamental to the development of acne, since such changes impair the barrier function of the skin, which in turn can cause inflammatory reactions. However, this mechanism is far from the only one capable of influencing the pathogenesis of acne with the participation of SSL.16
To determine the functional state of the skin, the determination of the main components ensuring the barrier properties of the skin—FFAs, CERs, and the relative amount of unsaturated and saturated FFAs—was carried out. Patients with acne showed a significant deviation in the amount of FFAs in the skin compared with healthy volunteers – the level of saturated FFAs was reduced, the level of unsaturated FFAs was increased, and the length of the FFA chains practically did not differ. In addition, there was a significant decrease in the average chain length of the CERs in the affected skin. Thus, it is these quantitative changes in the composition of the skin that led to a decrease in the barrier function and the appearance of acne.17
To determine the severity of changes in the barrier function of the skin, the determination of TEWL was performed, as a result of which it turned out that the TEWL value in patients with acne is expected to be higher compared with healthy volunteers. The results of these studies correlate with other studies of changes in the barrier function of the skin in various pathologies. Acne-related skin dysfunctions are also directly dependent on the lipid composition of skin cells and on the quantitative and relative levels of CERs, FFAs and cholesterol – the main components of the skin that provide its barrier properties.18
Conclusions
Lipidomic analysis can help more deeply understand the cause of various skin diseases of various origins. As has been shown in the studies described, often the main cause of inflammatory skin reactions is a decrease in the barrier function of the skin. This decrease is caused by a change in the qualitative and quantitative lipid composition of the skin, regardless of the initial reasons that affected such changes. In patients with inflammatory skin processes, there is a decrease in the overall level of CERs and an insufficient supply of FFAs in cells, which inhibits many enzymatic processes in the skin cells, disrupting their proliferation and differentiation. Due to abnormal cellular mechanisms, the skin cannot properly perform its functions. Having studied the differences in the composition of the affected skin from healthy individuals, new strategies can be developed for treating or maintaining diseases in the remission stage. It can be either anti-inflammatory therapy or replacement therapy, which consists in delivering the necessary substances and enzymes to the skin cells in order to fill in the missing components and normalize the cellular processes with subsequent improvement of the functional properties of the skin.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a Sechenov University grant.Notes and references
- J. van Smeden, M. Janssens, G. S. Gooris and J. A. Bouwstra, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2013, 1841, 295–313 CrossRef PubMed
.
- K. Sandra and P. Sandra, Curr. Opin. Chem. Biol., 2013, 17, 847–853 CrossRef CAS PubMed
.
- F. Bonté, P. Pinguet, J. M. Chevalier and A. Meybeck, J. Chromatogr. B: Biomed. Sci. Appl., 1995, 664, 311–316 CrossRef
.
- T. Sadowski, C. Klose, J. Gerl Mathias, A. Wójcik-Maciejewicz, R. Herzog, K. Simons, A. Reich and M. A. Surma, Sci. Rep., 2017, 7, 43761 CrossRef PubMed
, 1–11.
- L. Norlén, I. Nicander, B. Rozell, S. Olmar and B. Forslind, In Vivo J. Invest. Dermatol., 1999, 112, 72–77 CrossRef PubMed
.
- P.-A. Wendling and G. Dell'Acqua, Skin Res. Technol., 2003, 9, 331–338 CrossRef PubMed
.
- D. Y. Leung, Allergol. Int., 2013, 62, 151–161 CrossRef CAS
.
- J. van Smeden, M. Janssens, G. S. Gooris and J. A. Bouwstra, Biochim. Biophys. Acta, 2014, 1841, 295–313 CrossRef CAS
.
- J. Ishikawa, H. Narita, N. Kondo, M. Hotta, Y. Takagi, Y. Masukawa, T. Kitahara, Y. Takema, S. Koyano, S. Yamazaki and A. Hatamochi, J. Invest. Dermatol., 2010, 130, 2511–2514 CrossRef CAS
.
- M. Janssens, J. van Smeden, G. S. Gooris, W. Bars, G. Portale, P. J. Caspers, R. J. Vreeken and T. Hankemeiser, J. Lipid Res., 2012, 53, 2755–2766 CrossRef CAS
.
- J. van Smeden, M. Janssens, W. A. Boiten, V. van Drongelen, L. Furio, R. J. Vreeken, A. Hovnanian and J. A. Bouwstra, J. Invest. Dermatol., 2014, 134, 1238–1245 CrossRef CAS
.
- J. H. Shin, J. C. Shon, K. Lee, S. Kim, C. S. Park, E. C. Choi, C. H. Lee, H. S. Lee and K. H. Liu, Anal. Bioanal. Chem., 2014, 406, 1917–1932 CrossRef CAS PubMed
.
- S. H. Moon, J. Y. Kim, E. H. Song, M. K. Shin, Y. H. Cho and N. I. Kim, Ann. Dermatol., 2013, 25, 321–326 CrossRef CAS PubMed
.
- X. Li, C. He, Z. Chen, C. Zhou, Y. Gan and Y. Jia, J. Cosmet., Dermatol. Sci. Appl., 2017, 16(2), 168–173 CrossRef PubMed
.
- M. Souvik, R. Mitra, A. Maitra, S. Gupta, S. Kumaran, A. Chakrabortty and P. P. Majumderb, Sci. Rep., 2014, 6, 36062 Search PubMed
.
- O. A. Bakry, R. M. El Shazly, S. M. El Farargy and D. Kotb, Indian Dermatol. Online J., 2014, 5, 9–16 CrossRef PubMed
.
- Y. Gan, M. Zhou, C. He, Z. Chen and Y. Jia, Br. J. Dermatol., 2018, 179, 732–740 CrossRef PubMed
.
- A. Yamamoto, K. Takenouchi and M. Ito, Arch. Dermatol. Res., 1995, 287(2), 214–218 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2019 |
