Synthesis and antitumor activity of fluorouracil – oleanolic acid/ursolic acid/glycyrrhetinic acid conjugates†
Abstract
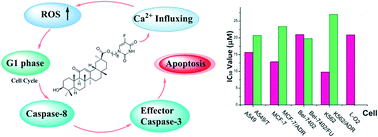
Due to the obvious adverse effects of 5-fluorouracil that limit its clinical usefulness and considering the diverse biological activities of pentacyclic triterpenes, twelve pentacyclic triterpene-5-fluorouracil conjugates were synthesized and their antitumor activities were evaluated. The results indicated that all the single substitution targeted hybrids (7a–12a) possessed much better antiproliferative activities than the double substitution targeted hybrids (7b–12b). Hybrid 12a exhibited good antiproliferative activities against all the tested MDR cell lines. Furthermore, it was revealed that 12a could induce intracellular calcium influx, the generation of ROS, arrest the cell proliferation at the G1 phase, and activate the apoptotic signaling caspase-8, which eventually activates the apoptotic effector caspase-3 and causes the later nuclear apoptosis.


 Please wait while we load your content...
Please wait while we load your content...