Photophysical properties and application in live cell imaging of B,B-fluoro-perfluoroalkyl BODIPYs†
Abstract
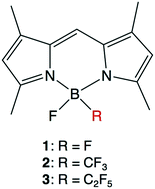
The photophysical properties of newly identified B,B-fluoro-perfluoroalkyl BODIPYs (2 and 3), which possess a fluoro group and a trifluoromethyl or pentafluoroethyl group at the boron center, were investigated. B,B-Fluoro-perfluoroalkyl BODIPYs 2 and 3 exhibited better photophysical/chemical properties than B,B-difluoro-BODIPY 1, as follows: (1) higher photostability both in methanol solvent and in a live cell environment, (2) higher stability against acid degradation, and (3) improved fluorescence signal-to-noise ratios in a cell system. These favorable properties of B,B-fluoro-perfluoroalkyl BODIPYs are likely due to the highly electron-withdrawing nature of the perfluoroalkyl groups on the boron atom, which reduces the reactivity to 1O2 and strengthens the complexation of the dipyrromethene ligands to the boron atom. Thus, B,B-fluoro perfluoroalkyl BODIPYs may be useful functional molecules for various applications.
- This article is part of the themed collection: Molecular Pharmacology


 Please wait while we load your content...
Please wait while we load your content...