An integrated microfluidic system for rapid detection and multiple subtyping of influenza A viruses by using glycan-coated magnetic beads and RT-PCR†‡
Abstract
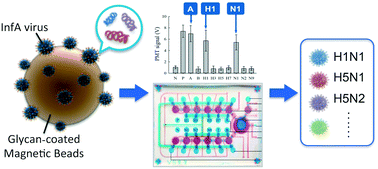
The influenza A (InfA) virus, which poses a significant global public health threat, is routinely classified into “subtypes” based on viral hemagglutinin (HA) and neuraminidase (NA) antigens. Because there are nearly 200 viral subtypes, current diagnostic approaches require multiplexing or array systems to cover various subtypes of HA and NA. A microfluidic chip featuring a HA × NA array was consequently developed herein for diagnosis and subtyping of InfA viruses via the use of glycan-coated magnetic beads followed by reverse transcription (RT) polymerase chain reaction (PCR). Up to 12 InfA subtypes were simultaneously detected in an automated fashion in less than 100 minutes on this microfluidic platform, representing a significant improvement in analysis speed compared to benchtop RT-PCR and chip-based microarray systems. The limits of detection of the RT-PCR assays ranged from 40 to 3000 copy numbers for the different subtypes of InfA viruses, around two orders of magnitude higher than in previous studies using microfluidic technologies. In summary, the array-type microfluidic chip system provides a rapid, sensitive, and fully automated approach for detection and multiple subtyping of InfA.
- This article is part of the themed collection: Coronavirus articles - free to access collection


 Please wait while we load your content...
Please wait while we load your content...