Development of a microdevice for facile analysis of theophylline in whole blood by a cloned enzyme donor immunoassay†
Abstract
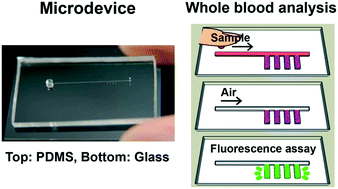
We have developed a microdevice for therapeutic drug monitoring. In this device, dispensing of sample and reagent was accomplished by simple manual operation of a syringe. Moreover, for a simple and rapid measurement, we used cloned enzyme donor immunoassay as a detection principle. These features and the reagent that is enclosed in microdevice beforehand make it possible to complete the facile analysis. In this paper, our model analyte was 1,3-dimethylxanthine (theophylline), a kind of bronchodilator. The fluorescence measurement of theophylline in whole blood was achieved with the limit of detection of 0.73 μg mL−1. This microdevice provides rapid analysis (4 min), requires only a small volume of sample (2 μL) and features simple operation; hence, it is readily applicable to point of care testing.


 Please wait while we load your content...
Please wait while we load your content...