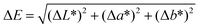Open Access Article
Open Access ArticleRecycling of vat and reactive dyed textile waste to new colored man-made cellulose fibers†
Simone
Haslinger
 a,
Yingfeng
Wang
a,
Marja
Rissanen
a,
Miriam Beatrice
Lossa
b,
Marjaana
Tanttu
c,
Elina
Ilen
c,
Marjo
Määttänen
d,
Ali
Harlin
d,
Michael
Hummel
a,
Yingfeng
Wang
a,
Marja
Rissanen
a,
Miriam Beatrice
Lossa
b,
Marjaana
Tanttu
c,
Elina
Ilen
c,
Marjo
Määttänen
d,
Ali
Harlin
d,
Michael
Hummel
 a and
Herbert
Sixta
a and
Herbert
Sixta
 *a
*a
aDepartment of Bioproducts and Biosystems, Aalto University, P. O. Box 16300, Espoo, FI-00076, Aalto, Finland. E-mail: simone.haslinger@aalto.fi; yingfeng.wang@aalto.fi; marja.rissanen@aalto.fi; michael.hummel@aalto.fi; herbert.sixta@aalto.fi
bDepartment of Chemistry and Biology, Hochschule Fresenius, University of Applied Science, Limburger Str. 2, 65510 Idstein, Germany. E-mail: lossa.miriam@stud.hs-fresenius.de
cDepartment of Design, Aalto University, Otaniementie 13, 02150 Espoo, Finland. E-mail: marjaana.tanttu@aalto.fi; ilena.elin@aalto.fi
dVTT Technical Research Centre of Finland Ltd, P.O. Box 1000, Espoo, FI-02044 VTT, Finland. E-mail: marjo.maattanen@vtt.fi; ali.harlin@vtt.fi
First published on 18th September 2019
Abstract
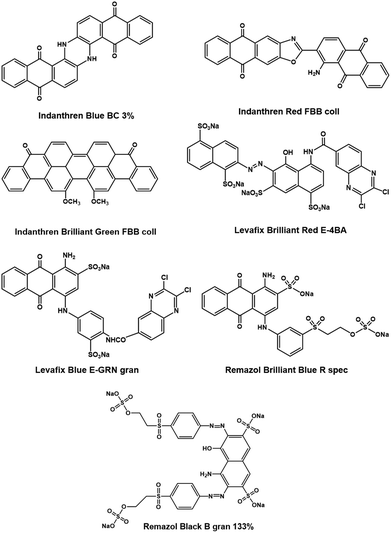
The successful recycling of colored textile waste and reuse of respective dyes would represent a major milestone of global efforts to reduce the environmental impact of the textile industry. The chemical upcycling of dyed pre- and postconsumer cotton waste is promoted by studying the spinability and color fastness of seven vat and reactive dyes (i.e. Indanthren Blue BC 3%, Indanthren Red FBB coll, Indanthren Brilliant Green FBB coll, Levafix Brilliant Red E-4BA, Levafix Blue E-GRN gran, Remazol Brilliant Blue R spec, and Remazol Black B 133%) during dry-jet wet spinning. Apart from the fabrics dyed with Levafix Brilliant Red E-4BA, all samples dissolved in 1,5-diazabicyclo[4.3.0]non-5-ene actetate, a superbase based ionic liquid, and could be converted to new colored man-made cellulose fibers. It was found that there is a clear discrepancy between the recyclability of dyed pre- and postconsumer cotton waste, resulting in significantly higher fiber properties up to tenacities of 59.8 cN/tex and elongations of 13.1% in case of the latter. All recycled fibers displayed a noticeable color change in the CIELab space (ΔE = 8.8–25.6) throughout the spinning process. Despite these deviations, almost all fibers and demo fabrics produced thereof exhibited bright colors that can be reused in textile industry. Only Remazol Black B 133% did not sufficiently translate to the new textile product. The wash and rubbing fastness of the fabrics knitted from the regenerated fibers was superior to the dyed waste fabrics mainly because of the homogenous distribution of the dyes along the fiber cross-section.
Introduction
Fast fashion has transformed the perception of clothing from a valuable, long-lasting product into a short-term use, disposable good.1 Europe only recycles 25% of its textile waste, the vast majority is incinerated or landfilled. Cotton is considered a renewable resource, but its ecological footprint is equal or even larger than the impact of polyester.2 When cotton is however recycled, its carbon footprint can be cut into half as further cotton cultivation is minimized.3Various strategies for the conversion of cotton waste garments into new value added products have been developed over the past decades. They can mainly be divided into chemical and mechanical approaches. In the latter, cotton waste is shredded, opened, and carded to reclaim single cotton fibers.4 Dependent on their final application, these can be pretreated or bleached before they are respun into new yarns.5 However, strategies to preserve and reuse the original color are urgently needed to avoid the generation of further effluents such as wastewater from dyeing.6 Textile dyeing can require up to 150 liters of water per kilogram of fabric. In developing countries, where most of the production takes place and where adequate environmental legislation is often lacking, the wastewater is frequently discharged unfiltered into waterways. 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of synthetic dyes are produced worldwide. Up to 200
000 tons of synthetic dyes are produced worldwide. Up to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of these dyes are lost to effluents every year during dyeing and finishing operations of textiles, due to the inefficiency of the dyeing processes.7
000 tons of these dyes are lost to effluents every year during dyeing and finishing operations of textiles, due to the inefficiency of the dyeing processes.7
In the recent past, some mechanical recycling technologies have emerged, aiming at color retention during the reuse of textile waste. The mechanical recovery of cotton circumvents both, cotton cultivation and dyeing, by sorting the raw-material prior to the yarn manufacturing. The waste material is subjected to a process similar to ginning to clean and open the cotton fibers.8 By blending different colors, a broad spectrum of yarns can be obtained, which show properties similar to conventional yarns produced from virgin materials.9 The drawback is that the raw-material consists of mainly pre-consumer cotton waste, whereas only small amounts of postconsumer cotton can be added to the yarn production.4,9 Nowadays, 75% of pre-consumer cotton is reused,3 while postconsumer waste is more problematic to recycle due to coloring, impurities and degradation induced by sun light irradiation, mechanical abrasion, and laundering.10,11
Therefore, chemical recycling offers more options for postconsumer waste. Recent studies have shown that cotton from textiles can be used to produce biogas,12 cellulose nanoparticles,13 aerogels14 or microcrystalline cellulose.15 When it comes to textile industry, a cradle-to-cradle approach is more desirable, implying that textile waste is dissolved and re-spun into new fibers. In 2016, we demonstrated the conversion of white post-consumer cotton to Lyocell type fibers by dry-jet wet spinning using the ionic liquid 1,5-diazabicyclo[4.3.0]non-5-ene actetate [DBNH] [OAc] as a direct solvent. The procedure first requires assessing the viscoelastic properties of the resulting solution, which are entirely determined by the cotton waste material. Dependent on how heavily the fabric was used and washed, its properties might be suitable for dry-jet wet spinning without any further pretreatment.16 In many cases, the degree of polymerization of cellulose, however, needs to be lowered by hydrolysis to a target value to fit the requirements of the spinning process.16,17 Besides the adjustment of macromolecular properties, the pretreatment can also involve purification steps such as bleaching and the removal of metals.18 A bleached product has so far been more preferable on a commercial scale due to hygiene and aesthetic reasons, although it increases the consumption of chemicals and water, also demanding subsequent re-dyeing. It is evident that these approaches do not sufficiently comply with the principles of green processing. A recycling strategy is hence only truly sustainable if the original color of a waste fabric can be translated to the new product directly.
Preliminary studies have shown that it is, in principle, possible to dissolve dyed textile waste and to spin new colored fibers thereof. This can be achieved via different systems such as the viscose process,19 the NMMO-based Lyocell process,20 alkali/urea,21 or a mixture of 1-butyl-3-methylimidazolium acetate and DMSO.22 Similarly, [DBNH] [OAc] can be used to recycle colored textile waste.23 However, there are more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different dyes used in textile manufacturing and identifying the exact chemical compound(s) in textile waste is nearly impossible.24 Therefore, more systematic studies are needed to discern categories of textile dyes that are suitable for chemical textile recycling. Respective dyes have to maintain their molecular integrity when exposed to the chemical environment and thermal impact of the fiber spinning process. Further, they should not alter the rheological properties of the spin dope, which could impair its subsequent spinability.25 Finally, the dyes have to coagulate with the cellulose to avoid their accumulation in the coagulation bath, which would affect the solvent recycling procedure and compromise the overall economy of the process. This recycling route, in which the dye is co-dissolved with the polymer, can be compared to spin dyeing, which is frequently used for synthetic polymers. Spun-dyed fibers have a considerably reduced environmental impact compared to classical textile dyeing.26 Initial studies have reported the spin dyeing of Lyocell fibers through the addition of inorganic pigments,27 different colorants and their precursors28 to the spinning solution. Also vat-dyed pulp mixed with undyed pulp was found to result in a good colorfastness.29,30
000 different dyes used in textile manufacturing and identifying the exact chemical compound(s) in textile waste is nearly impossible.24 Therefore, more systematic studies are needed to discern categories of textile dyes that are suitable for chemical textile recycling. Respective dyes have to maintain their molecular integrity when exposed to the chemical environment and thermal impact of the fiber spinning process. Further, they should not alter the rheological properties of the spin dope, which could impair its subsequent spinability.25 Finally, the dyes have to coagulate with the cellulose to avoid their accumulation in the coagulation bath, which would affect the solvent recycling procedure and compromise the overall economy of the process. This recycling route, in which the dye is co-dissolved with the polymer, can be compared to spin dyeing, which is frequently used for synthetic polymers. Spun-dyed fibers have a considerably reduced environmental impact compared to classical textile dyeing.26 Initial studies have reported the spin dyeing of Lyocell fibers through the addition of inorganic pigments,27 different colorants and their precursors28 to the spinning solution. Also vat-dyed pulp mixed with undyed pulp was found to result in a good colorfastness.29,30
Vat dyes are water insoluble in their oxidized form, but dissolve in water once reduced. This enables them to be precipitated on the fiber surface by a sequence of reduction and oxidation reactions. Moreover, vat dyes, such as Indanthrenes, are thermally stable due the presence of anthraquinone units. In the 1960s, 40–50% of all cellulosics were dyed with vat dyes because of their excellent resistance to acid, alkali, and bleaching. Nowadays, vat dyeing is still used for textile products that require to withstand a large number of washing cycles or industrial laundering, including work wear, household articles, and outdoor equipment. With the development of more stable and cost-effective reactive dyes, vat dyes lost their dominant position on the market. Today, the global share of reactive dyes amounts 20–30%, which involves the dyeing of most cellulose products. Reactive dyes consist of four units, most commonly referred to as solubilizing, chromophore, bridging, and reactive groups.31 Comparable to vat-dyes, reactive dyes with anthraquinone chromophores are considered to be more resistant to laundering, elevated temperatures, and bleaching than azo colorants.32,33 The reactive groups of reactive dyes can mainly be divided into halo heterocycles that form a covalent bond with cellulose via nucleophilic substitution, and activated vinyl compounds such as vinyl sulfones that attach to cellulose through addition reactions.31 Finally, the reactivity of these units and the nature of the chromophore determine the color fastness of a garment throughout its lifecycle as well as its subsequent recyclability.
As mentioned before, no studies have yet systematically investigated the role of different dye classes in a chemical upcycling process for cotton based textile waste, which nonetheless appears essential to prevent an extensive consumption of chemicals and water. For the first time, this study targets to create a better understanding of the recyclability of dyed cotton fabrics within a dry-jet wet spinning process, using a superbase based ionic liquid such as [DBNH] [OAc] (Ioncell™), by assessing the impact of seven commercial colorants onto the production of man-made cellulose fibers. We tested three vat dyes (Indanthren Blue BC 3%, Indanthren Red FBB coll, Indanthren Brilliant Green FBB coll) and four reactive dyes, among them two vinyl sulfone dyes (Remazol Brilliant Blue R spec, Remazol Black B 133%), and two halo quinoxaline dyes (Levafix Brilliant Red E-4BA, Levafix Blue E-GRN gran). Pre- and post-consumer cotton waste was used as textile substrate.
The white fabrics were dyed, ground, and their intrinsic viscosities were measured. This approach was chosen because the exact dye formulation of waste textiles is usually unknown. In this way, it was possible to relate the process behavior and color stability to the particular dye during the entire spinning process.
The pre-consumer fabric was pretreated with electron beam irradiation, sulfuric acid and endoglucanases to adjust the degree of polymerization. The effect of the pretreatment was assessed based on the molar mass distributions, and compared to the properties of untreated dyed post-consumer waste. Subsequently, the samples were subjected to dry-jet wet spinning and if spinable processed to knitted fabrics. Besides the determination of tensile properties and fiber orientation, the color change of the dyes throughout the spinning process was monitored, together with their subsequent fastness to washing and rubbing. Ultimately, final prototype garments were manufactured from staple fibers spun directly from solutions of worn dyed textile fabrics to demonstrate that the dyeing of white textile waste corresponds to that of genuine dyed textile waste in terms of recyclability.
Materials and methods
Raw material
White pre-consumer cotton (100%) was purchased from Iisalmen Kangastukku (Finland). The product name was Painopohja (printing base) and consisted of a plain weave structure (148 g m−2). White postconsumer hospital bed sheets (100% cotton) were supplied by the Uusimaa Hospital Laundry (Uudenmaan Sairaalapesulo Oy, Finland). As all samples displayed distinct viscoelastic properties, the limiting viscosity of each bed sheet was determined separately according to the SCAN-CM 15:88 standard of cellulose in cupri-ethylenediamine (CED) solution. Moreover, all samples were ground using a Wiley Mill and their dry-matter content was determined.Dyes and dyeing procedures
Indanthrene Red FBB coll (C.I. Vat Red 10), Indanthren Brilliant Green FBB coll (C.I. Vat Green 1), Levafix Brilliant Red E-4BA (C.I. Reactive Red 158), and Levafix Blue E-GRN gran (C.I. Reactive Blue XX) were kindly provided by DyStar (Germany). Indanthren Blue BC 3% (C.I. Vat Blue 6), Remazol Brilliant Blue R spec (C.I. Reactive Blue 19) and Remazol Black B gran 133% (C.I. Reactive Black 5) were purchased from A. Wennström Oy (Finland). The chemical structures of the dyes can be found in Fig. 1.All fabrics were dyed in 1 kg batches. During vat-dyeing, water (20 l) was heated in a kettle (50 °C) and Na2S2O4 (3 g ml−1), 25 wt% sodium hydroxide (NaOH) and Na2SO4·10H2O (12 g l−1) were added. Once the respective Indanthrene dye (2% of the dry weight of the samples) had been dissolved and reduced, the cotton fabrics were soaked in the dyeing bath for 45 min under constant stirring. In order to enhance the oxidation of the dyes, the fabrics were subsequently rinsed with water followed by hydrogen peroxide (2 ml l−1) until no leaching of color could be observed anymore. Reactive dyeing was conducted in a washing machine (Esteri Pesukoneet Oy, Vantaa, Finland) using a 60 °C/20 l program with the following dyeing sequence: Prewetting + gentle spin–dyeing–rinsing–boiling–rinsing–spin. As in the previous procedure, the reactive dyes amounted 2% of the dry mass of the cotton samples. The sample to liquor ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (kg l−1). Na2SO4·10H2O (50 g l−1) was added to increase the affinity of the dyes to cotton, while Na2CO3 (9 g l−1) was used to provide an alkaline environment. After dyeing, all fabrics were air-dried.
20 (kg l−1). Na2SO4·10H2O (50 g l−1) was added to increase the affinity of the dyes to cotton, while Na2CO3 (9 g l−1) was used to provide an alkaline environment. After dyeing, all fabrics were air-dried.
Pretreatment of pre-consumer cotton
Waste cotton was pretreated in three different ways using acid hydrolysis (A), electron beam irradiation (e-beam), and sequences of enzymatic treatment with endoglucanases (EG) and acids (A) to reduce the degree of polymerization (DPv) to an intrinsic viscosity of 450 ± 50 ml g−1 (DPv = 1050 ± 100).(A): Sulfuric acid (H2SO4, 95–97%, Merck or Sigma) was diluted to 0.27 M and heated to 82 °C. Subsequently, ground cotton was added in a sample to liquor ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and treated 7–9 min to yield a target viscosity of 450 ± 50 ml g−1. After the treatment, the sample was washed with cold deionized water to stop the reaction.
100 and treated 7–9 min to yield a target viscosity of 450 ± 50 ml g−1. After the treatment, the sample was washed with cold deionized water to stop the reaction.
(E-beam): The electron beam pretreatment was conducted by means of a 10 MeV accelerator at Leoni Studer AG (Switzerland). A series of different electron beam dosages (5–30 kGy) were tested to reach the required viscosity.
(EG-A, A-EG): The sequences of endoglucanase and acid treatments were carried out by VTT (Finland). The shredded vat-dyed cotton material was treated with an endoglucanase-rich enzyme Ecopulp R (AB Enzymes, Finland) at a consistency of 25 wt% for 4 h at 50 °C. Prior the treatment, the cotton sample was dispersed in water to 2 wt% and subsequently dewatered to enhance the enzymatic treatment. The enzyme dosage was 10 mg protein per g of cotton pulp. After the reaction had been stopped (90 °C, 30 min), the fibrillated material was cooled and rinsed with deionized water. The acid treatment was carried out for 2 h at 90 °C at a consistency of 10 wt% (oven dry cotton/water). The pre-heated cotton material was dispersed in water and 0.45 wt% (based on the oven dry weight of cotton) of H2SO4 (0.5 M, FF-Chemicals Ab) were added.
After the reaction had been stopped by diluting the mixture with deionized water to 5 wt% consistency, the sample was dewatered and washed with cold deionized water.
Molar mass distribution (MMD)
The molar mass distribution (MMD) of both dyed pre- and post-consumer samples was analyzed by gel permeation chromatography applying one precolumn (PLgel Mixed-A, 7.5 × 50 mm) and four analytical columns (PLgel Mixed-a, 7.5 × 300 mm). The set-up was equipped with a refractive index detector (Shodex RI-101). The samples were dissolved in 90 g l−1 LiCl/N,N-dimethylacetamide (DMAc) via a solvent exchange procedure (H2O/acetone/DMAc). The system was operated at a temperature of 25 °C and a flow rate of 0.75 ml min−1 using 9 g l−1 LiCl/DMAc as an eluent. The injection volume was 100 μl. Pullulan standards (Mw 343–708![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) were used as calibration standards employing a direct-standard calibration model.34
000 Da) were used as calibration standards employing a direct-standard calibration model.34
[DBNH] [OAc]
1,5-Diazabicyclo [4.3.0] non-5-ene (99%, Fluorochem, UK) was neutralized with glacial acetic acid (100%, Merck, Germany).35Dissolution studies
In order to analyze the solubility of the dyes in the ionic liquid, small amounts of dye were suspended in [DBNH] [OAc] and stirred under constant heating (80°, 1 h). A drop of the resulting mixture was then placed onto a glass slide and observed under a heating microscope (80 °C, Zeiss Axio scope A1, Zeiss AG, Germany). The same procedure was also conducted for the spinning dopes prepared from the dyed waste cotton fabrics to evaluate the effect of dyes on the dissolution of the respective cotton substrate.Dope preparation and rheology
After grinding, the dyed fabrics were dissolved in [DBNH] [OAc] (80 °C, 90 min) to obtain a 13% cellulose solution using a vertical kneader system. After dissolution, undissolved particles were removed from the solution by press filtration (80 °C, 1–2 MPa, metal filter fleece, 5–6 μm absolute fineness, Gebr. Kufferath AG, Germany). The resulting dope was then shaped into a cylindrical mold and stored upon solidification (1–7 days, 5–8 °C). The viscoelastic properties of the spinning solution were evaluated on an Anton Paar Physica MCR 300 rheometer and on an Anton Paar Physica MCR 302 rheometer (Austria). A dynamic frequency sweep test (100–0.1 s−1) was used to obtain the complex viscosity η* and the dynamic moduli (G′, G′′) as a function of the angular frequency ω. Assuming the validity of the Cox-Merz rule, an apparent zero shear viscosity η0* of the spinning solutions could be calculated, and the crossover point of G′ and G′′ was determined to yield additional information on the cellulose substrate.36Dry-jet wet spinning
The cellulose solution was spun into filaments on a customized lab-scale piston unit (Fourne Polymertechnik, Germany). The spin dope was heated according to its rheological properties and extruded through a 200-holes spinneret (0.1 mm diameter, 0.02 mm capillary length). While passing a ca. 1 cm air gap, the filaments were stretched with a draw ratio of 12 (DR = vtake-up/vextr.). After spinning, the fibers were cut into staple fibers (4 cm), opened, washed with water (80 °C, 2 h), and air dried.Tensile properties and birefringence
The properties of all fibers spun from colored post-consumer cotton waste including tenacity (cN/tex), elongation at break (%), and linear density (dtex) were determined on a Favigraph device (Textechno, Germany). Test parameters: 20 cN load cell, 10 mm gauge length, 10 mm min−1 test speed, 100 mg pretension, fiber count 20. All samples were measured in conditioned (20 °C, 65% relative humidity) and wet state (10 s wetting prior to the testing). The Young's Modulus was calculated from the initial slope of the individual stress strain curves. The fibers produced from pretreated, dyed pre-consumer cotton were analyzed on Vibroskop and Vibrodyn 400 devices (Lenzing, Instrument, Austria). Test parameters: 20 mm gauge length, 20 mm min−1 test speed, 100 mg pretension, fiber count: 10.The total orientation of the all fibers was estimated using a polarizing light microscope (Zeiss Axio Scope, Zeiss AG, Germany). Fibers showing an average linear density were taped to a glass slide, and the birefringence angle was measured for the bottom, middle, and top part of the fibers. Assuming a density of 1.5 g cm−3, the total orientation factor ft could be calculated by dividing Δn by the maximum birefringence value of cellulose (0.062).37
Color coordinates and color difference
The optical properties of all samples before and after spinning were assessed by CIELab coordinates using a 10 °C observer and a standard illuminant D65 (SpectroScan, GretagMacbeth, Germany). The difference in color ΔE was calculated from the following equation,where ΔE denotes the total difference between two colors in the CIELab space, ΔL* the difference in lightness, Δa* the difference in the red-green axis, and Δb* the difference in the yellow-blue axis.
Yarn spinning and tensile testing
The staple fibers were soaked into a spin finishing solution (50 °C, 5 min) consisting of Aflian CVS and Leomin PN (Archroma, Switzerland). The ratio of Aflian CVS and Leomin PN was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in total amounting 0.25% of the dry mass of the fibers. The sample to liquor ratio was 1
1, in total amounting 0.25% of the dry mass of the fibers. The sample to liquor ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. After drying, the fibers were opened by a fiber opener (Trash analyzer281C, Mesdan, Italy). The opened fibers were carded (Carding Machine 337A, Mesdan, Italy for the fibers spun from post-consumer waste or Automatex, Italy for the fibers spun from pre-consumer cotton). The carded web was formed to a sliver, drafted (Stiro Roving Lab 3371, Mesdan, Italy) and doubled twice, and formed into a roving. The yarn was spun with a ring-spinning machine (Ring Lab 82BA, SER.MA.TES, Italy, for the fibers spun from post-consumer waste, or Ring Lab, Mesdan, Italy, for the fibers spun from pre-consumer cotton). The yarn twist direction was Z and twist per meter 700. Both tenacity (cN/tex) and elongation at break (%) of the spun yarns (single thread) were measured on a MTS 400 tensile tester (MTS, USA) with a 50 N load cell, a gauge length of 250 mm, and a test speed of 250 mm min−1. Before the measurement, the yarns were conditioned (20 ± 2 °C, 65 ± 2% relative humidity) and their average linear density (tex = g per 1000 m) was determined from 20 m skeins. The plied yarn was produced by twisting two threads together (DirectTwist®, Agteks, Turkey). The ply twist direction was S with 300 twists per meter.
20. After drying, the fibers were opened by a fiber opener (Trash analyzer281C, Mesdan, Italy). The opened fibers were carded (Carding Machine 337A, Mesdan, Italy for the fibers spun from post-consumer waste or Automatex, Italy for the fibers spun from pre-consumer cotton). The carded web was formed to a sliver, drafted (Stiro Roving Lab 3371, Mesdan, Italy) and doubled twice, and formed into a roving. The yarn was spun with a ring-spinning machine (Ring Lab 82BA, SER.MA.TES, Italy, for the fibers spun from post-consumer waste, or Ring Lab, Mesdan, Italy, for the fibers spun from pre-consumer cotton). The yarn twist direction was Z and twist per meter 700. Both tenacity (cN/tex) and elongation at break (%) of the spun yarns (single thread) were measured on a MTS 400 tensile tester (MTS, USA) with a 50 N load cell, a gauge length of 250 mm, and a test speed of 250 mm min−1. Before the measurement, the yarns were conditioned (20 ± 2 °C, 65 ± 2% relative humidity) and their average linear density (tex = g per 1000 m) was determined from 20 m skeins. The plied yarn was produced by twisting two threads together (DirectTwist®, Agteks, Turkey). The ply twist direction was S with 300 twists per meter.
Demonstration products
The yarn produced from recycled, Indanthren Blue BC 3% dyed pre-consumer cotton was employed to produce the baby jacket demonstrator. The base fabric of the baby jacket was interlock-knit (127 g m−2) using a single thread yarn (18 tex Z 700), and the sleeve cuffs were 2 × 1 rib-knit using two combined threads together (18 tex Z 700 × t0). The fabrics were knitted by a flatbed weft knitting machine (Stoll CMS ADF 32W E7.3 multi gauge, Stoll, Germany). Both materials were finished solely by steam ironing before sewing. The binding material in the edges was a commercial knitted viscose, narrow fabric band. The cotton based sewing yarns are typical in knit garment production. The garments were sewed in a prototyping laboratory using industrial-like sewing machinery. No finishing treatments were applied after sewing.The blue, plied yarn (221 tex Z 700 × 2 S 300) produced from recycled Remzol Brilliant Blue R spec dyed post-consumer cotton was used for the scarf demonstrator. The knit structure was mixed purl and lace and the same flatbed knitting machine was employed as in the baby jacket demonstrator.
For color fastness testing, single jersey fabrics (150 g m−2) were knitted (Stoll CMS ADF 32W E7.3 multi gauge, Stoll, Germany) from plied yarns spun from pre- and post-consumer cotton.
Wash fastness
The color fastness of the dyed fabrics and the fibers after spinning was tested according to EN ISO 105-C06 (color fastness to domestic and commercial laundering). The specimens were sewn together with a DW-multifiber adjacent fabric. If the specimen was loose fibers, the mass of loose fiber was equal to one-half of the combined mass of the adjacent fabrics (multifiber and non-dyeable fabrics). The size of cotton fabric specimen was 100 mm × 40 mm. The mass of regenerated cellulosic fabric specimens was similar to cotton fabric specimens. The wash liquor was prepared by dissolving 4 g l−1 of detergent (AATCC 1993 Standard Reference Detergent WOB) in deionized water. The test method was A1S, where the temperature was 40 °C and the liquor volume was 150 ml. The number of steel balls was 10 and the washing time was 30 min. The specimens were washed in the Linitest equipment (Original Hanau, Germany). At the end, the specimens were rinsed twice for 1 min in two separate 100 ml portions of water at 40 °C. The specimens were air-dried. The change in color of the specimen and the staining of the multifiber fabric were assessed using the grey scales under D65 illumination. The highest value of the grey scale is 5 (no color change or staining) and 1 is the worst (cf. Fig. S1 and S2 in the ESI†).Rubbing test
The color fastness to rubbing of the dyed fabrics and the regenerated cellulosic knitted fabrics was tested according to the EN ISO 105-X12:2016 standard. The specimens were rubbed 20 times (10 times to and 10 times fro) with a dry rubbing cloth (desized, bleached cotton cloth) and with a wet (100% pick-up of water) rubbing cloth. The diameter of the rubbing finger was 16 mm with a downward force of 9 N. The test cloth was removed from the finger and air-dried. The staining of the test cloth was assessed with the grey scale under D65 illumination.Recycling of fibers from worn denim fabrics to a scarf
In comparison to the recycling of self-dyed pre-consumer cotton waste, a used denim fabric was dissolved directly in [DBNH] [OAc] and spun into new staple fibers, which were then processed into a scarf for demonstration purposes. The individual process steps are described in the ESI (Fig. S7 and Tables S4–S6†).Results and discussion
Raw-material and pretreatment
Discarded textiles can be classified as pre- and post-consumer waste. The former is produced during the manufacturing of textiles and represents, for example, excess material resulting from cutting clothing patterns. As its properties are usually well known, pre-consumer cotton shows a significantly higher recyclability than post-consumer garments that have undergone a whole lifecycle. When establishing a recycling strategy for cotton rich textiles, it is essential to know the origin of the product. The molecular properties of pre-consumer cotton resemble virgin one with a high intrinsic viscosity of up to 2000 ml g−1. At the same time, these fibers might contain a higher amount of dyes and finishing chemicals. Wearing and laundering subsequently decrease the chemicals present in the textile product, but also affect the macromolecular molecular properties of cellulose such as the degree of polymerization.10,11 This implies that the cotton's viscosity decreases dependent on the usage, eventually creating a very heterogeneous post-consumer product.In order to study the recyclability of dyed textile waste systematically and to gain a better understanding of the associated challenges, both pre-consumer and post-consumer cotton were used as raw material. The fabrics where then dyed as described in the Materials and methods section to obtain substrates that resemble real waste textiles, but with exact knowledge on the type and amount of dye in the fabric.
As reported previously, cotton waste needs to show a limiting viscosity of 400–500 ml g−1 to be spun to Lyocell-type fibers using NMMO monohydrate or [DBNH] [OAc] as solvent.16 Therefore, the pre-consumer fabric was subjected to different pretreatments, such as electron beam irradiation (e-beam), H2SO4 hydrolysis (A), and combinations of acid washing with H2SO4 (A) and enzymatic hydrolysis with endoglucanase (EG), after vat-dyeing with Indanthren Blue BC 3%. The idea of the pretreatments was to adjust the degree of polymerization (DP) of cellulose to a target DP to obtain the viscoelastic properties necessary for optimal spinning while maintaining the original color of the fabric. Mechanical treatments such as disk refining or chemicals that would cause bleaching (i.e. O3 or H2O2) were thus avoided.
Sequences of acid washing and endoglucanase treatments have yet successfully been employed on other waste materials.38 Whereas acids most commonly degrade cellulose to a polydispersity index (PDI) close to 2.0,39 enzymes such as endoglucanase result in a broadening of the molar mass distribution and enhance the cellulose's reactivity towards the dissolving agent.40 In general, a broad molar mass distribution of the cellulose substrate can be beneficial for the spinning process because it contains both long chains for fiber strength and shorter ones to act as fillers.41 Therefore, enzymatic treatments are expected to result in a better spinnability than acid treatments only. A combination of both is done for cost efficiency and to remove possible contaminations by metals. Adjusting the viscosity by e-beam irradiation appears more sustainable in terms of wastewater generation and saves costs emerging from drying after wet-treatment. During e-beam irradiation, high-energy electrons (10 MeV) penetrate a stack of sheets of cellulose materials (fabric) with almost constant energy. The resulting radicals are statistically distributed in the material, which leads to a uniform degradation of the cellulose chains. Due to unsaturated, stable radicals and the formation of labile groups such as carbonyl groups, depolymerization reactions may continue even after the e-beam treatment, especially under alkaline and/or oxidative conditions.42
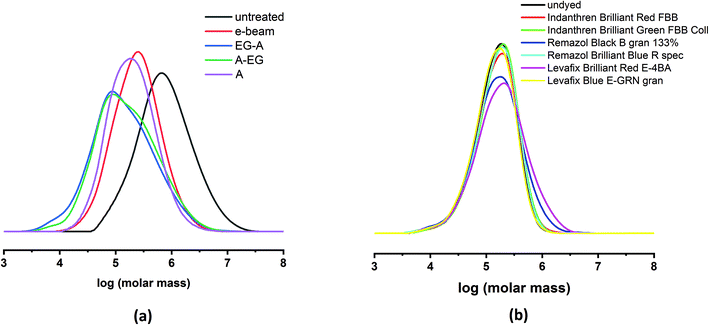
A summary of the studied treatments and their influence on the limiting viscosity and molar mass distribution is displayed in Fig. 2a and Table 1.
| Sample | Pretreatment | Viscosity/(ml g−1) | M n/kDa | M w/kDa | PDI | DP < 100/% | DP > 2000/% |
|---|---|---|---|---|---|---|---|
| Pre-consumer | |||||||
| Untreated | — | 2032 ± 50 | 423.1 ± 1.0 | 1417.3 ± 57.7 | 2.4 ± 1.4 | 0 | 78.1 ± 0.8 |
| Indanthren Blue BC 3% | e-beam | 545 ± 11 | 147.3 ± 3.2 | 391.1 ± 10.6 | 2.7 ± 0.1 | 0.3 ± 0.1 | 37.8 ± 0.4 |
| Indanthren Blue BC 3% | EG-A | 496 ± 15 | 67.1 ± 7.6 | 304.5 ± 13.4 | 4.6 ± 0.7 | 3.6 ± 1.3 | 24.8 ± 0.5 |
| Indanthren Blue BC 3% | A-EG | 470 ± 12 | 75.9 ± 1.2 | 380.0 ± 55.9 | 5.0 ± 0.8 | 2.7 ± 0.2 | 28.1 ± 0.5 |
| Indanthren Blue BC 3% | A | 454 ± 11 | 114.5 ± 14.5 | 314.9 ± 6.1 | 2.8 ± 0.4 | 0.9 ± 0.9 | 29.4 ± 0.7 |
| Post-consumer | |||||||
| Undyed | — | 396 ± 16 | 94.1 ± 4.2 | 193.8 ± 2.6 | 2.1 ± 0.1 | 1.5 ± 0.4 | 15.3 ± 0.2 |
| Indanthren Brilliant Red FBB | — | 461 ± 78 | 93.7 ± 1.5 | 206.5 ± 3.5 | 2.2 ± 0.02 | 1.4 ± 0.06 | 17.7 ± 0.3 |
| Indanthren Brilliant Green FBB Coll | — | 435 ± 33 | 100.1 ± 4.0 | 210.5 ± 1.0 | 2.1 ± 0.1 | 1.3 ± 0.3 | 18.5 ± 0.02 |
| Remazol Black B gran 133% | — | 448 ± 113 | 91.8 ± 0.1 | 255.3 ± 0.1 | 2.8 ± 0.003 | 1.9 ± 0.08 | 23.1 ± 0.2 |
| Remazol Brilliant Blue R spec | — | 422 ± 63 | 91.9 ± 2.5 | 204.0 ± 0.5 | 2.2 ± 0.1 | 1.8 ± 0.2 | 17.5 ± 0.04 |
| Levafix Brilliant Red E-4BA | — | 465 ± 28 | 102.4 ± 5.3 | 308.3 ± 1.5 | 3.0 ± 0.1 | 1.6 ± 0.4 | 29.1 ± 0.2 |
| Levafix Blue E-GRN gran | — | 426 ± 56 | 89.1 ± 1.8 | 186.8 ± 0.3 | 2.1 ± 0.04 | 1.7 ± 0.3 | 14.3 ± 0.01 |
The initial viscosity of the Indanthren dyed pre-consumer fabric ranged around 2032 ml g−1 and was decreased by e-beam irradiation, acid treatment (A), as well as by EG-A and A-EG sequences to 545, 454, 496, and 470 ml g−1, respectively. Both e-beam and A only had a minor effect on the polydispersity index (PDI) of the samples (2.7 and 2.8). Despite the similar PDI, it is evident that A tends to degrade rather longer polymer chains than shorter ones, which is consequently reflected in the lower amount of DP > 2000 chains (29.4%) compared to the statistical cleavage induced by e-beam irradiation (37.8%). In contrast, both EG-A and A-EG sequences substantially increased the PDI of the cotton substrate from 2.4 to 4.6 and 5.0 also leading to a rise in the low molecular weight fractions (DP < 100) with 3.6% and 2.7%, respectively.
The aim for the post-consumer waste was to be used without any pretreatment to exclude possible effects thereof on the dye structures. All post-consumer samples consisted of white hospital bed sheets supplied by a laundering company. It can be assumed that most samples had a similar lifetime, which resulted in similar molecular properties. In an initial assessment, we determined the limiting viscosities of 50 bed sheets to estimate their overall suitability for dry-jet wet spinning in [DBNH] [OAc]. The predefined target range for the viscosities was 350–550 ml g−1. Samples below or beyond this range were not considered for further processing. The viscosity distribution of the analyzed samples is illustrated in Fig. 3.
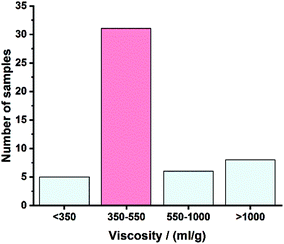 | ||
| Fig. 3 Viscosity distribution of the 50 hospital bed sheets analyzed; in red, the number of samples chosen for dry-jet wet spinning. | ||
Only 10% (5) of the bed sheets displayed viscosities below 350 ml g−1, while 28% (14) showed values above 550 ml g−1. The remaining 62% (31) of the samples were found adequate to be used in the following experiments.
As stated in the Experimental section, bed sheets of varying viscosities were then combined to 1 kg batches, and subsequently dyed with a selection of Indanthren, Remazol, and Levafix dyes. Their average molar mass distributions and viscosities can be found in Fig. 2b and Table 1. The viscosities of the samples varied from 396 ml g−1 (undyed) to 465 ml g−1 (Levafix Brilliant Red E-4BA).
The PDI of the bed sheets dyed with Remazol Black B gran 133% and Levafix Brilliant Red E-4BA was noticeably higher with 2.8 and 3.0 compared to the undyed reference (2.1) and the remaining dyed samples (2.1–2.2). From the molar mass distributions, it can be inferred that both samples, Remazol Black B gran 133% and Levafix Brilliant Red E-4BA, contained a significantly higher amount of high molecular weight fractions (23.1% and 29.1%) than the rest of the post-consumer batches (14.3%–18.5%). Remazol and Levafix are reactive dyes, which covalently bind to cellulose, whereas Indanthren dyes are precipitated on the cotton fiber. An effect on the molar mass distribution caused through dyeing cannot be excluded, but seems unlikely in respect to the obvious heterogeneity of post-consumer textile waste.
Dissolution of cotton and dry-jet wet spinning
The formation of cellulosic filaments from ionic liquid solutions depends on the liquid thread's ability to withstand capillary and deformation forces.43 During the spinning process, the cellulose solution is extruded through small nozzles, where the cellulose molecules are pre-oriented. Further orientation is induced by stretching of the resulting filaments in the air-gap.44 The applied draw subsequently aligns the cellulose molecules coaxially leading to increased breaking tenacities and lower linear densities (i.e. titers), which are required for textile applications. The stretchability of a polymer solution is yet dependent on its rheological properties. Consequently, the zero shear viscosities η0* and the dynamic moduli (G′, G′′) at their crossover point enable to predict the spinnability of the cotton solutions (cf.Table 2, and for more details Table S2, Fig. S3 as well as Fig. S4 in the ESI†).| Sample | Trials | Cotton/wt% | Spin dope rheology | DR | Fiber properties | Conditioned | Wet | Birefringence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/°C | η 0*/Pa s | ω/(1 s−1) | G′ = G′′/Pa | Fiber yield/wt% | Titer/dtex | Tenacity/(cN/tex) | Elongation/% | Tenacity/(cN/tex) | Elongation/% | Δn | f total | ||||
| Pre-consumer | |||||||||||||||
| Indanthren Blue BC 3% e-beam | 3 | 13–14 | 70 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.07 | 6996 | 12 | 0–17 | 1.4 ± 0.2 | 40.3 ± 2.7 | 7.4 ± 1.4 | 33.7 ± 3.3 | 7.25 ± 1.03 | 0.048 ± 0.007 | 0.78 ± 0.12 |
| Indanthren Blue BC 3% EG-A | 12 | 13–14 | 70 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.53 | 2179 | 12 | 20–74 | 1.2 ± 0.1 | 44.3 ± 1.4 | 10.8 ± 0.6 | 39.5 ± 3.2 | 12.5 ± 0.7 | 0.048 ± 0.007 | 0.78 ± 0.11 |
| Indanthren Blue BC 3% A-EG | 1 | 13 | 70 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.61 | 1630 | 12 | 13 | 1.0 ± 0.1 | 45.5 ± 2.8 | 8.9 ± 1.1 | 45.0 ± 3.5 | 11.5 ± 1.3 | 0.044 ± 0.017 | 0.72 ± 0.27 |
| Indanthren Blue BC 3% A | 2 | 13 | 70 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.90 | 3905 | 12 | 0–25 | 1.2 ± 0.1 | 48.5 ± 4.1 | 12.8 ± 1.3 | 47.4 ± 4.1 | 12.4 ± 3.1 | 0.044 ± 0.004 | 0.71 ± 0.06 |
| Post-consumer | |||||||||||||||
| Undyed | 1 | 13 | 65 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.42 | 4554 | 12 | 34 | 1.2 ± 02 | 56.4 ± 4.3 | 13.0 ± 1.4 | 53.4 ± 4.0 | 13.7 ± 1.1 | 0.043 ± 0.004 | 0.70 ± 0.07 |
| Indanthren Brilliant Red FBB | 1 | 13 | 65 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.76 | 4913 | 12 | 39 | 1.4 ± 0.4 | 54.4 ± 8.4 | 14.3 ± 1.0 | 54.3 ± 2.3 | 14.3 ± 1.1 | 0.045 ± 0.006 | 0.72 ± 0.09 |
| Indanthren Brilliant Green FBB Coll | 1 | 13 | 65 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.37 | 3484 | 12 | 42 | 1.3 ± 0.2 | 54.7 ± 4.4 | 12.3 ± 1.9 | 51.2 ± 6.0 | 13.2 ± 1.5 | 0.044 ± 0.005 | 0.71 ± 0.09 |
| Remazol Black B gran 133% | 1 | 13 | 65 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.29 | 4319 | 12 | 42 | 1.4 ± 0.4 | 50.7 ± 8.8 | 13.1 ± 1.4 | 51.4 ± 2.6 | 14.2 ± 1.5 | 0.038 ± 0.010 | 0.61 ± 0.16 |
| Remazol Brilliant Blue R spec | 1 | 13 | 65 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.18 | 3131 | 12 | 49 | 1.2 ± 0.2 | 59.8 ± 4.1 | 13.1 ± 1.6 | 57.0 ± 4.4 | 15.1 ± 2.1 | 0.047 ± 0.004 | 0.75 ± 0.07 |
| Levafix Brilliant Red E-4BA | 1 | 13 | 65 | 812![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
n.a. | n.a. | 12 | 0 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Levafix Blue E-GRN gran | 1 | 13 | 65 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
0.51 | 4317 | 12 | 43 | 1.2 ± 0.2 | 56.0 ± 4.7 | 13.1 ± 1.9 | 54.3 ± 4.3 | 13.8 ± 1.8 | 0.047 ± 0.015 | 0.76 ± 0.22 |
Viscoelastic solutions usually show a Newtonian plateau at lower shear rates until shear thinning occurs due to increased shear stress.45 Supposing that the Cox Merz rule is valid, this behavior allows the calculation of the zero shear viscosity η0* using the Carreau or the Cross model.36η0* denotes the viscosity of a polymer solution upon no shear stress applied. It is directly affected by the concentration and temperature within the spinning process. In previous studies, we could show that a η0* between 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–30
000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pas indicates the optimum spinning temperature if standard dissolving pulp is employed.35,46 However, these values deviate once waste materials such as paper or cotton are dissolved.16,38,47
000 Pas indicates the optimum spinning temperature if standard dissolving pulp is employed.35,46 However, these values deviate once waste materials such as paper or cotton are dissolved.16,38,47
As depicted in Table 2, the average zero shear viscosities of the pre-consumer samples ranged between 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400–31
400–31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 Pas at 70 °C, while η0* for post-consumer cotton was significantly higher with 32
100 Pas at 70 °C, while η0* for post-consumer cotton was significantly higher with 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700–78
700–78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 Pas at optimum spinning temperatures around 65 °C. This demonstrates that pre- and postconsumer cotton waste show different ideal spinning temperatures and zero shear viscosities dependent on origin and pretreatment, and might thus demand different processing conditions.
200 Pas at optimum spinning temperatures around 65 °C. This demonstrates that pre- and postconsumer cotton waste show different ideal spinning temperatures and zero shear viscosities dependent on origin and pretreatment, and might thus demand different processing conditions.
Presumably, this deviation is due to a difference in the molar mass distribution of the samples. The crossover point (COP) of storage and loss modulus (G′, G′′) is influenced by the amount of long and short polymer chains present in the spin dope, as well as by the overall viscosity of the cellulose sample. The narrower the molar mass distribution, the lower is the substrate's polydispersity index, and the higher is the COP (G′ = G′′). Moreover, lower limiting viscosities of the cellulose pulp shift the COP to higher angular frequencies ω.48 In particular, the enzyme pretreated pre-consumer cotton (Indanthren Blue BC 3% A-EG and Indanthren Blue BC 3% EG-A) displayed noticeably lower average values for the G′ = G′′ (1630–2179 Pa) than the remaining pre- and postconsumer samples (3131–6996 Pa). These results correspond to the molar mass distributions depicted in Table 1 and in Fig. 2a & b. Inevitably, these properties affected the spinnability of the respective cotton solutions, which is reflected in the fiber yields summarized in Table 2.
The highest yields could be obtained with pre-consumer Indanthren Blue BC 3% EG-A (20–74%). Neither electron beam radiation nor A led to quantitative fiber amounts (0–25%). It is unclear whether this discrepancy can solely be attributed to the narrow molar mass distribution,41 or to a lack of optimization. Despite similar COPs, all spinnable post-consumer samples resulted in yields between 34–49% at 5 °C lower spinning temperatures. An exception was Levafix Brilliant Red E-4BA, which was not spinnable at all because of its obvious gel character implied by the absence of the COP. In this context it also needs to be noted that fiber yields can only be regarded as tentative indicators for a substrate's spinnability as the whole study was conducted on a lab-scale equipment, where material losses cannot be avoided because of batch processing. Despite the high yields reached with the EG-A batches, it is evident that the post-consumer samples showed higher yields on average. The spinning quality would thus better be described by the maximum draw ratio extruded filaments can withstand; its assessment was however without the scope of this study.
The resulting fiber properties (see Table 2 and Fig. 4) show a considerable difference between the spun pre- and post-consumer samples. As mentioned in the experimentals, all fibers were stretched with a draw ratio of 12, which means that the take-up velocity of the godets collecting the fibers is 12 times higher than the extrusion velocity of the spin dope. At similar concentrations (13–14 wt%), this leads to fibers with comparable linear densities (1.0–1.4 dtex). The variation within this parameter usually results from differences in the solubility of the cotton pulp during the dissolution stage, which finally influences the real concentration of the cotton solution. It was observed that untreated cotton pulp dissolved better than pre-treated one, which formed undissolved aggregates in the spin dope presumably due to hornification after wet processing and drying.
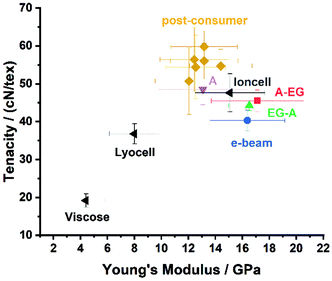
The breaking tenacities and elongations of the spun post-consumer cotton (50.4–59.0 cN/tex, 12.3–14.3%) in conditioned state were significantly higher than the values of the pre-consumer samples (40.3–48.5 cN/tex, 7.4–12.8%). This trend also continued in wet state (51.2–54.3 cN/tex, 13.2–14.2% for post-consumer; 39.5–47.4 cN/tex, 11.5–12.5% for pre-consumer), and is reflected in the Young's Modulus of the fibers (see Fig. 4 and Table S3,† as well as average stress strain curves in Fig. S5 & S6†). A similar trend for the total orientation of the fibers could not be observed (cf.Table 2).
Although all fibers exceeded the properties of commercial Lyocell and Viscose, the recycled post-consumer fibers appeared on average stronger and more flexible than the ones produced from pre-consumer waste, exhibiting Young's Moduli from 12.0–14.4 GPa and 13.1–17.0 GPa, respectively. Moreover, the post-consumer samples yielded also 6–25% better tenacities compared to the Ioncell fibers spun from bleached pre-hydrolysis kraft pulp. This also corresponds to the Moduli of Toughness obtained for all regenerated post-consumer fibers displaying values of 60.0–69.5 MPa, whereas the pre-consumer samples only yielded moduli up to 53.6 MPa (cf. Table S3 in the ESI†).
These results agree with earlier studies describing the recycling of cotton waste in NMMO or [DBNH] [OAc].16,17,20 The choice of the dye did not appear to have an impact on the fiber properties apart from Levafix Brilliant Red E-4BA, which was not spinnable due to gel formation.
Yarn spinning
All batches of the spun Indanthren Blue BC 3% dyed pre-consumer cotton were combined for the yarn spinning process. All other samples were processed to yarns separately. The fibers were spin finished to control their antistatic and cohesion properties in the yarn spinning process. The strength of the spun yarns depends on the fiber strength and the frictional resistance to slippage.49 The angle of fiber inclination increases with increasing yarn twist. At the same time, the component of fiber strength in the yarn axis decreases. Otherwise, the frictional resistance between the fiber raises as the yarn twist is increased until the fiber slippage in the yarn is eliminated. In the short-staple yarn spinning, 30–70% of the fiber strength can be exploited in the yarn.50 The properties of the spun yarns are summarized in Table 3. Similar to the fiber properties, the yarn spun from Indranthren Blue BC 3% yielded the lowest tenacity 25.0 cN/tex with an elongation at break of 7.6%, while the dyed post-consumer samples showed tenacities of 28.8–32.7 cN/tex and elongations of 6.7–7.1%.| Sample | Titer/tex | Tenacity/(cN/tex) | Elongation/% |
|---|---|---|---|
| Pre-consumer | |||
| Indanthren Blue BC 3% | 17.8 ± 1.5 | 25.0 ± 4.7 | 7.6 ± 0.9 |
| Post-consumer | |||
| Indanthren Brilliant Red FBB | 21.7 ± 1.7 | 28.8 ± 3.8 | 6.9 ± 0.5 |
| Indanthren Brilliant Green FFB Coll | 20.8 ± 1.8 | 28.9 ± 3.6 | 6.4 ± 0.5 |
| Remazol Black B gran 133% | 25.3 ± 0.5 | 29.0 ± 3.4 | 7.1 ± 0.5 |
| Remazol Brilliant Blue R spec | 21.2 ± 1.0 | 32.7 ± 3.9 | 6.9 ± 0.5 |
| Levafix Blue E-GRN gran | 21.5 ± 0.9 | 31.4 ± 4.4 | 6.7 ± 0.6 |
Fabrics and demonstration products
All fabrics used for the wash fastness and rubbing tests as well as the demonstration products (see Fig. 5) were knitted from the yarns listed above as discussed in earlier publications.35,51 The baby jacket was manufactured using the samples obtained from Indanthren Blue BC 3%, whereas the scarf was knitted from Remazol Brilliant Blue R spec dyed samples. The fabrics below represent the remaining samples produced from the recycled fibers on the right and the dyed waste fabrics on the left.Dyes in the spinning process
Whether the original color of a waste fabric can be reused in new textile fibers depends on a variety of factors. Concerning post-consumer waste, the dye needs to exhibit a good color fastness towards washing and rubbing so that the waste garment still shows a sufficient color intensity at the end of its lifecycle. When recycled in a dry-jet wet spinning process, dyes additionally require to tolerate the chemical environment thereof, including elevated temperatures and pH, and should not change the rheological properties of the spin dope.25,52 Indanthren Blue BC 3%, Indanthren Red FBB coll, and Indanthren Brilliant Green FBB coll represent vat dyes, which have already been studied as colorants for spin dyeing in the Lyocell process yielding excellent color fastness.28–30 Their oxidized form is usually insoluble in water, which makes it possible to precipitate them on the fiber surface through a sequence of reduction and oxidation reactions. Vat dyes also show a high thermal stability due their aromatic structure formed by antraquinone units. Likewise, reactive dyes with antraquinone (Remazol Brilliant Blue R spec or Levafix Blue E-GRN gran) have been reported to be particularly durable towards laundering, elevated temperatures, and bleaching32,33 especially compared to azo chromophores (Levafix Brilliant Red and Remazol Black B gran 133%). Remazol dyes exist as mono (Remazol Brilliant Blue R spec) and bifunctional colorants (Remazol Black B gran 133%) and attach to the ionized cellulose via etherification of the vinyl sulphone groups.53 Competing hydrolysis reactions can however cause a restrained substantivity.54 On the contrary, Levafix dyes form covalent bonds with cellulose through a nucleophilic substitution reaction of their halo quinoxaline units. They contain two reactive groups and may form two dye-fiber bonds. Their reactivity is lower compared to dichloro-s-triazine dyes and they might undergo hydrolysis reactions in acidic environment.55 Finally, the stability of a dye within a recycling process strongly depends on its chemical structure, the nature of its interaction with cellulose and the solvent.In initial dissolution experiments (cf. Table S1 in the ESI†), we found all dyes apart from Indanthren Blue BC 3% and Indanthren Brilliant Red FBB, soluble in [DBNH] [OAc]. The same applied for almost all dyed cotton fabrics, excluding the sample dyed with Levafix Brilliant Red E-4BA, where aggregations of undissolved fibers could be observed under the microscope. The insolubility of the two Indanthren dyes, however, did not affect the quality of the resulting spin dopes. The dyes and cellulose formed a homogenous solution in the ionic liquid, which led to regenerated fibers that displayed a uniform distribution of dye throughout the fiber cross-section.27,30 Only Remazol dyes leached into the spin bath causing color fading, most likely due to insufficient fixation during the dyeing step.31Table 4 summarizes the CIELab parameters lightness L*, a* (red-green axis), and b*(yellow-blue axis) as well as the overall color difference ΔE in the CIELab space. ΔE is a tool to assess the similarity of colors during quality control. Values of 0–0.5 describe no change, 0.5–1.0 an almost imperceptible change (usually not visible by the human eye), 1.0–2.0 a small color difference, 2.0–4.0 a perceivable difference, and 4.0–5.0 a rarely tolerated change, whereas values above 5.0 denote a different color.
| Samples | Waste fabric | Recycled regenerated fibers | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||
| Indanthren Blue BC 3% | 69.0 ± 0.3 | −4.9 ± 0.3 | −22.2 ± 1.1 | 56.6 ± 1.1 | −6.6 ± 0.1 | −14.0 ± 0.3 | 14.9 |
| Indanthren Brilliant Red FBB | 40.5 ± 1 | 49.1 ± 0.6 | 6.9 ± 0.4 | 39.5 ± 0.8 | 38.1 ± 0.7 | 12.9 ± 0.3 | 12.6 |
| Indanthren Brilliant Green FBB Coll | 37.0 ± 0.5 | −46.9 ± 0.4 | −1.4 ± 0.1 | 34.5 ± 0.4 | −38.4 ± 0.1 | −0.9 ± 0.1 | 8.8 |
| Remazol Black B gran 133% | 18.7 ± 0.3 | −5.0 ± 0.1 | −16.2 ± 0.2 | 35.6 ± 1.2 | 15.6 ± 0.3 | 6.2 ± 0.2 | 25.6 |
| Remazol Brilliant Blue R spec | 43.0 ± 0.6 | −1.8 ± 0.1 | −39.7 ± 0.3 | 56.9 ± 3.1 | 0.7 ± 0.7 | −20.7 ± 0.4 | 23.6 |
| Levafix Brilliant Red E-4BA | 41.9 ± 0.3 | 62.0 ± 0.3 | 0.01 ± 0.2 | n.a. | n.a. | n.a. | n.a. |
| Levafix Blue E-GRN gran | 37.5 ± 0.9 | −1.5 ± 0.1 | −31.97 ± 0.3 | 35.3 ± 1.4 | −3.5 ± 0.5 | −23.3 ± 0.6 | 9.2 |
All dyes showed a noticeable color difference (ΔE = 8.8–25.6), with Indanthren dyes (ΔE = 8.8–14.) and Levafix (ΔE = 9.2) resulting in similar fastness properties. During the dissolution of cotton, [DBNH] [OAc] creates an alkaline environment that enables the conversion of vat dyes into their reduced form. This can induce breakages of the hydrogen bonds between cellulose and the respective dyes, which can affect the final shade of the regenerated fibers. On the contrary, an alkaline cleavage of the ether bond between cellulose and the Levafix dyes seems rather unlikely.
The color leaching of Remazol Brilliant Blue R spec and Black B gran 133% was followed by the largest alterations (ΔE = 23.6; 25.6). As displayed in Fig. 6, the absorbance maximum of Remazol Brilliant Blue R spec did not experience any significant shift (590 nm to 587 nm) throughout the spinning process, while the absorbance band of Remazol Black B 133% shifted from 597 nm to 574 nm. According to the Color Index, Remazol Black B 133% (C.I. Reactive Black 5) exhibits a rather poor alkali resistance, but it remains unclear whether the strong color change can be attributed to a degradation of the chromophore in [DBNH] [OAc], or to an insufficient fixation of dye during the dyeing step.
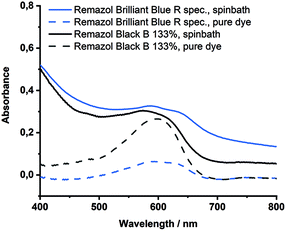 | ||
| Fig. 6 UV spectra of the spinbath after spinning with Remazol Blue R spec and Remazol Black B 133%, as well as of the pure dyes in water. | ||
Moreover, the spinning process triggered an increase of b* (yellow-blue axis) in all samples, and raised the lightness L* of the Remazol dyed samples, while the remaining samples (i.e. Levaifx, Indanthren) showed a slight decrease of L*. For a* (red-green axis), no particular trend could be observed.
Without a doubt, any recycling process that consists of reshaping polymeric material also brings about color changes. When cotton is dissolved and subsequently coagulated, its crystalline structure transforms from cellulose I to cellulose II. Besides, the dissolution and regeneration is also accompanied by a change of the surface morphology of the regenerated fibers.16 The result is an increased luster, which for example becomes evident in the color of the fibers.
Eventually, it can be assumed that the observed color changes are a combination of surface phenomena, insufficient boil-off after dyeing, as well as chemical reactions occurring in the alkaline environment of [DBNH] [OAc]. Apart from Remazol Black B 133% and Levafix Brilliant Red E-4BA, all dyed waste fabrics nonetheless translated to brightly colored fibers that certainly can be employed in textile industry (cf.Fig. 5).
Color fastness to washing and rubbing
The color fastness to washing and color fastness to rubbing are the most typical color fastness tests for textiles. They predict how much color fades and stains white garments during the laundering or rubbing. Vat dyes (Indanthren) have good all-round fastness properties because of their water insolubility. Reactive dyes (Levafix and Remazol) show in particular good wet fastness due to covalent bonding between the dye molecule and cellulose. Table 5 summarizes the wash fastness results of all dyed waste fabrics and the fabrics manufactured from the dry-jet wet spun fibers.| Sample | Change in colour | Numerical rating for staining | |||||
|---|---|---|---|---|---|---|---|
| Wool | Acrylic | Polyester | Polyamide | Cotton | Cellulose acetate | ||
| Cotton | |||||||
| Indanthren Blue BC 3% | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Indanthren Brilliant Red FBB | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Indanthren Brilliant Green FBB Coll | 5 | 4–5 | 5 | 5 | 5 | 5 | 5 |
| Remazol Black B gran 133% | 4–5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Remazol Brilliant Blue R spec | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Levafix Brilliant Red E-4BA | 5 | 5 | 5 | 5 | 5 | 4–5/5 | 5 |
| Levafix Blue E-GRN gran | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Ioncell (from recycled cotton waste) | |||||||
| Indanthren Blue BC 3% | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Indanthren Brilliant Red FBB | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Indanthren Brilliant Green FBB Coll | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Remazol Black B gran 133% | 4–5/5R | 5 | 5 | 5 | 5 | 5 | 5 |
| Remazol Brilliant Blue R spec | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Levafix Brilliant Red E-4BA | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Levafix Blue E-GRN gran | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
As described before, Remazol Brilliant Blue R spec is a monofunctional vinyl sulphone dye. Although it forms a covalent bond with cellulose, it also undergoes a competing hydrolysis reaction with water during the dyeing process. The same applies for Remazol Black B gran, but it shows a higher fixation rate because of two reactive groups. Similarly, the dichloroquinoxaline dyes Levafix Brilliant Red E-4BA and Levafix Blue E-GRN gran can partially hydrolyze during dyeing and this may reduce their wash fastness.55 However, sufficient rinsing and boiling-off after dyeing removes the hydrolyzed dyes completely. This study demonstrated an excellent wash fastness for both cotton and regenerated cellulosic fabrics, regardless of the dyes employed (cf.Table 5).
Table 6 describes the rubbing fastness of the fabrics before and after recycling. The low rubbing fastness of the dyed waste fabrics arises from the superficial presence of the dye. It may cause the formation of heavy shades, inadequate washing at the end of dyeing, and the coloration of only a few molecules at the textile-air interface. Besides, it might affect the water solubility of the dyes and lead to a weak dye-fibre attachment on the surface layer. Overall, wet rubbing fastness tends to produce lower results compared to dry rubbing, which is due to the partly solubilization of the dye and its migration to the surface of colored fabric. Consequently, the regenerated cellulose fibers showed a significantly better rubbing fastness as the dyes were homogenously distributed in the fiber structure by the spinning process.
| Sample | Numerical rating of staining | |
|---|---|---|
| Dry | Wet | |
| Cotton | ||
| Indanthren Blue BC 3% | 4–5 | 2 |
| Indanthren Brilliant Red FBB | 4–5 | 2 |
| Indanthren Brilliant Green FBB Coll | 3–4 | 2 |
| Remazol Black B gran 133% | 2–3 | 1–2 |
| Remazol Brilliant Blue R spec | 4–5 | 3 |
| Levafix Brilliant Red E-4BA | 4 | 2 |
| Levafix Blue E-GRN gran | 5 | 3 |
| Ioncell (from recycled cotton waste) | ||
| Indanthren Blue BC 3% | 5 | 5 |
| Indanthren Brilliant Red FBB | 4–5 | 4 |
| Indanthren Brilliant Green FBB Coll | 4 | 3–4 |
| Remazol Black B gran 133% | 5 | 4–5 |
| Remazol Brilliant Blue R spec | 5 | 4–5 |
| Levafix Brilliant Red E-4BA | n.a. | n.a. |
| Levafix Blue E-GRN gran | 5 | 4–5 |
Recovery of the ionic liquid [DBNH] [OAc]
An entirely green recycling process demands the processing chemicals to be recyclable in the same manner as the waste substrate, which it upcycles. After spinning, the ionic liquid [DBNH] [OAc] accumulates in the aqueous coagulation bath. Therefore, the water must be removed from the ionic liquid to a tolerable residual content, while at the same time accumulated decomposition products of the solute must be eliminated. These approaches are part of our on-going research and will be published in more detail soon.Conclusions
We successfully demonstrated that it is possible to upcycle dyed pre- and post-consumer cotton waste to new man-made cellulose fibers retaining the color and having superior properties compared to commercial Viscose or Lyocell. The properties of the regenerated fibers were shown to be dependent on the origin of the waste substrate and the pretreatments employed. This is reflected in the tenacities and elongations at break of 40.3–48.5 cN/tex 7.4–12.8% for pretreated pre-consumer waste, and 50.7–59.8 cN/tex and 12.3–14.3% for untreated post-consumer cotton. Beside of Levafix Red E-4BA, the dyes did not affect the solubility of the cotton substrates in [DBNH] [OAc]. All translated colors changed throughout the spinning process (ΔE = 8.8–25.6) most likely due to alterations in the macromolecular structure of cellulose and leaching of the dyes into the spin bath (i.e.in particular Remazol Black B 133%). Nevertheless, most samples still displayed bright colors, which were suitable for the manufacturing of fabrics and demonstrator products. The wash and rubbing fastness increased after spinning as the colorants were incorporated homogenously into the fiber matrix. Eventually, the recyclability of dyed cotton will demand adequate labelling of the chemicals involved to enable proper reprocessing at the end of its lifecycle. It can be concluded that vat dyes and reactive dyes with anthraquinone units have the greatest potential to be re-used in dry-jet wet spinning. In terms of sustainability, colored post-consumer waste cotton requires less processing than pre-consumer fabrics as its molar mass distribution tends to be adjusted to fit the spinning parameters by wearing and laundering. Eventually, the manufacture of a scarf successfully demonstrated that worn jeans (i.e. denim fabrics) can be recycled into new, high-quality fibers that retain most of their color using the Ioncell™ process.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 646226. The authors also gratefully acknowledge Leena Katajainen (Aalto University) and VTT (Finland) for their support in material pretreatment, as well as Kaniz Moriam, Mikaela Trogen, Karl Mihhels, Yibo Ma, Sherif Elsayed and Hilda Zahra (Aalto University) for assisting in fiber spinning and dope preparation.References
- S. Bishop, Environ. Health Perspect., 2007, 115, A448–A454 Search PubMed.
- N. Cherrett, Ecological footprint and water analysis of cotton, hemp and polyester, Stockholm Environmental Institute, Stockholm, 2005 Search PubMed.
- S. S. Muthu, Y. Li, J. Y. Hu and L. Ze, Fibers Polym., 2012, 13, 1065–1070 CrossRef CAS.
- D. S. Heifetz, United States Pat, 53331801, 1994 Search PubMed.
- D. L. Ball and M. H. Hance, United States Pat, 5519925, 1995 Search PubMed.
- Ellen MacArthur Foundation, 2017, http://www.ellenmacarthurfoundation.org/publications.
- F. M. Drumond Chequer, G. A. R. de Oliveira, E. R. Anastacio Ferraz, J. Carvalho, M. V. Boldrin Zanoni and D. P. de Oliveir, in Eco-Friendly Textile Dyeing and Finishing, ed. M. Gunay, InTech, 2013 Search PubMed.
- HKRITA, Garment to Garment Recycling System, 2019 Search PubMed.
- F. A. Esteve-Turrillas and M. de La Guardia, Resour., Conserv. Recycl., 2017, 116, 107–115 CrossRef.
- A. Palme, A. Idström, L. Nordstierna and H. Brelid, Cellulose, 2014, 21, 4681–4691 CrossRef CAS.
- H. Wedin, M. Lopes, H. Sixta and M. Hummel, Text. Res. J., 2019, 10, 004051751984815 Search PubMed.
- A. Isci and G. N. Demirer, Renewable Energy, 2007, 32, 750–757 CrossRef CAS.
- T. Fattahi Meyabadi, F. Dadashian, G. Mir Mohamad Sadeghi and H. Ebrahimi Zanjani Asl, Powder Technol., 2014, 261, 232–240 CrossRef CAS.
- B. Zeng, X. Wang and N. Byrne, Carbohydr. Polym., 2019, 205, 1–7 CrossRef CAS PubMed.
- R. Xiong, X. Zhang, D. Tian, Z. Zhou and C. Lu, Cellulose, 2012, 19, 1189–1198 CrossRef CAS.
- S. Asaadi, M. Hummel, S. Hellsten, T. Härkäsalmi, Y. Ma, A. Michud and H. Sixta, ChemSusChem, 2016, 9, 3250–3258 CrossRef CAS PubMed.
- S. Björquist, J. Aronsson, G. Henriksson and A. Persson, Text. Res. J., 2017, 88, 2485–2492 CrossRef.
- S. Haslinger, M. Hummel, A. Anghelescu-Hakala, M. Määttänen and H. Sixta, Waste Manage., 2019, 97, 88–96 CrossRef CAS PubMed.
- A. Von der Eltz, United States Pat, US5609676, 1995 Search PubMed.
- L. V. Haule, C. M. Carr and M. Rigout, J. Cleaner Prod., 2016, 112, 4445–4451 CrossRef CAS.
- W. Liu, S. Liu, T. Liu, T. Liu, J. Zhang and H. Liu, Carbohydr. Polym., 2019, 206, 141–148 CrossRef CAS PubMed.
- Y. Ma, B. Zeng, X. Wang and N. Byrne, ACS Sustainable Chem. Eng., 2019, 11937–11943 CAS.
- E. Smirnova, Master's Thesis, Aalto University, 2017.
- M. A. Hassaan and A. El Nemr, Am. J. Environ. Sci. Eng., 2017, 1, 64–67 Search PubMed.
- B. Tawiah and B. K. Asinyo, Int. J. Manage., Inf., Technol. Eng., 2016, 4, 65–80 Search PubMed.
- (a) S. S. Muthu, Handbook of life cycle assessment of textiles and clothing, Woodhead Publishing, Oxford, 1st edn, 2015 Search PubMed; (b) N. Terinte, B. M. K. Manda, J. Taylor, K. C. Schuster and M. K. Patel, J. Cleaner Prod., 2014, 72, 127–138 CrossRef.
- H. Ruef, Austria Pat, EP121463B1, 2000 Search PubMed.
- P. Bartsch and H. Ruef, Austria Pat, WO99/46434, 1999 Search PubMed.
- T. Bechtold and A. P. Manian, Austria Pat, WO2007070904A1, 2006 Search PubMed.
- A. P. Manian, H. Ruef and T. Bechtold, Dyes Pigm., 2007, 74, 519–524 CrossRef CAS.
- N. N. Mahapatra, Textile dyes, WPI Publishing, New Delhi, 1st edn, 2016 Search PubMed.
- M. MA and G. Sun, Dyes Pigm., 2004, 63, 39–49 CrossRef CAS.
- P. Bigambo, H. Liu, P. J. Broadbent, C. M. Carr and M. Rigout, Dyes Pigm., 2018, 148, 91–98 CrossRef CAS.
- (a) R. Berggren, F. Berthold, E. Sjöholm and M. Lindström, J. Appl. Polym. Sci., 2003, 88, 1170–1179 CrossRef CAS; (b) M. Borrega, L. K. Tolonen, F. Bardot, L. Testova and H. Sixta, Bioresour. Technol., 2013, 135, 665–671 CrossRef CAS PubMed.
- A. Michud, M. Tanttu, S. Asaadi, Y. Ma, E. Netti, P. Kääriainen, A. Persson, A. Berntsson, M. Hummel and H. Sixta, Text. Res. J., 2015, 86, 543–552 CrossRef.
- R. J. Sammons, J. R. Collier, T. G. Rials and S. Petrovan, J. Appl. Polym. Sci., 2008, 110, 1175–1181 CrossRef CAS.
- J. Lenz, J. Schurz and E. Wrentschur, Holzforschung, 1994, 48, 72–76 CrossRef CAS.
- Y. Ma, M. Hummel, M. Määttänen, A. Särkilahti, A. Harlin and H. Sixta, Green Chem., 2016, 18, 858–866 RSC.
- P. J. Flory, Principles of Polymer Chemistry, Cornell University Press, S.l., 1953 (1969) Search PubMed.
- J. Pérez, J. Muñoz-Dorado, T. de La Rubia and J. Martínez, Int. J. Microbiol., 2002, 5, 53–63 CrossRef PubMed.
- A. Michud, M. Hummel and H. Sixta, Polymer, 2015, 75, 1–9 CrossRef CAS.
- H. Sixta, Handbook of pulp, Wiley-VCH, John Wiley, Chichester, Weinheim, 2006 Search PubMed.
- (a) A. Ziabicki and R. Takserman-Krozer, Kolloid-Z.u.Z.Polymere, 1964, 198 Search PubMed; (b) A. Ziabicki and R. Takserman-Krozer, Kolloid-Z.u.Z.Polymere, 1964, 199, 9–13 CrossRef CAS.
- H.-P. Fink, P. Weigel, H. J. Purz and J. Ganster, Prog. Polym. Sci., 2001, 26, 1473–1524 CrossRef CAS.
- X. Chen, Y. Zhang, L. Cheng and H. Wang, J. Polym. Environ., 2009, 17, 273–279 CrossRef CAS.
- H. Sixta, A. Michud, L. Hauru, S. Asaadi, Y. Ma, A. W. T. King, I. Kilpeläinen and M. Hummel, Nord. Pulp Pap. Res. J., 2015, 30, 43–57 CAS.
- Y. Ma, M. Hummel, I. Kontro and H. Sixta, Green Chem., 2018, 20, 160–169 RSC.
- T. G. Mezger, Applied rheology. With Joe Flow on rheology road, Anton Paar, Graz, 5th edn, 2018 Search PubMed.
- E. Oxtoby, Spun Yarn Technology, Elsevier Science, Kent, 2014 Search PubMed.
- W. Klein, The technology of short-staple spinning, 2nd edn, 1998, vol. 1 Search PubMed.
- M. Hummel, A. Michud, M. Tanttu, S. Asaadi, Y. Ma, L. K. J. Hauru, A. Parviainen, A. W. T. King, I. Kilpeläinen and H. Sixta, in Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials, ed. O. J. Rojas, Springer International Publishing, Cham, 2016, pp. 133–168 Search PubMed.
- A. S. Mahmoud, A. E. Ghaly and S. L. Brooks, Am. J. Biochem. Biotechnol., 2007, 3, 22–41 Search PubMed.
- A. J. Ahmed, Text. Dyer Printer, 1995, 19–24 CAS.
- D. M. Lewis, Color. Technol., 2014, 130, 382–412 CAS.
- M. Clark, Handbook of textile and industrial dyeing, Woodhead Publishing, Cambridge, UK, 2011, pp. 116–117 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9gc02776a |
| This journal is © The Royal Society of Chemistry 2019 |