Solvent basicity controlled deformylation for the formation of furfural from glucose and fructose†
Abstract
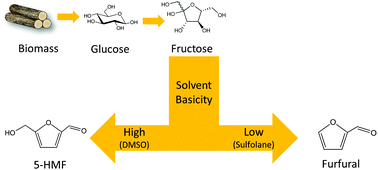
The conversion of glucose and fructose to furfural can improve the productivity of furfural synthesis from biomass. We report that sulfolane, a weakly basic polar aprotic solvent, promoted the formation of furfural from hexoses with a maximum yield of 50% obtained from fructose. Addition of Sn/SBA-15 that acted as a Lewis acid catalyst enabled the conversion of glucose to furfural with 36% yield. Analysis of products obtained from isotopically labelled glucose showed that furfural is produced by elimination of the C6 carbon atom as a formaldehyde molecule. DFT calculations revealed that this elimination reaction is plausible in the presence of weakly basic solvents that are unable to abstract the proton from the C–H bond in the last step of the reaction, which would otherwise lead to formation of 5-HMF. The furfural yield was correlated with the basicity of solvents, calculated as proton affinity (Epa), confirming the hypothesis that the basicity of solvent determines the selectivity for the formation of furfural or 5-HMF. Hence furfural formation from hexoses can be achieved by acid catalysed reaction of hexoses in the presence of low basicity polar aprotic solvents.
- This article is part of the themed collection: International Symposium on Green Chemistry 2019


 Please wait while we load your content...
Please wait while we load your content...