Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Production and purification of crystallized levoglucosan from pyrolysis of lignocellulosic biomass
Marjorie R.
Rover
 a,
Alvina
Aui
b,
Mark Mba
Wright
b,
Ryan G.
Smith
a and
Robert C.
Brown
a,
Alvina
Aui
b,
Mark Mba
Wright
b,
Ryan G.
Smith
a and
Robert C.
Brown
 *ab
*ab
aBioeconomy Institute, Iowa State University, Ames, IA, USA. E-mail: rcbrown3@iastate.edu; Tel: +1 515 294 7934
bDepartment of Mechanical Engineering, Iowa State University, Ames, IA, USA
First published on 2nd October 2019
Abstract
Levoglucosan has significant potential in commercial applications for the synthesis of polymers, solvents and pharmaceuticals. It is currently overlooked for commercial applications due to its high cost of synthesis and purification. We have developed a system to produce pure crystals of levoglucosan based on the fast pyrolysis of lignocellulosic biomass. A novel bio-oil recovery system concentrated levoglucosan along with other anhydrosugars, sugars and phenolic compounds in a non-aqueous “heavy ends” fraction. Liquid–liquid water extraction separated sugar-rich solubilized carbohydrates from non-soluble phenolic compounds. The solubilized carbohydrate fraction, contaminated with partially soluble phenolic monomers, was filtered through Sepabeads SP207 adsorption resin to produce clarified juice. The composition of the clarified juice on a dry basis after resin filtration and rotary evaporation was 81.2% sugars, 4.45–4.60% volatile non-sugar, 1.71% carboxylic acids and 12.5–12.6% unidentified compounds, which was sufficiently pure to crystallize the sugars by evaporation. A cold solvent rinse of the crystal mass separated and purified levoglucosan from other sugars. Levoglucosan purity was 102.5% ± 3.109% at the 99% confidence level. Techno-economic analysis of a plant pyrolyzing 250 tonne per day of pretreated biomass to produce cellulosic sugars indicated a minimum selling price (MSP) for pure levoglucosan crystals of $1333 per MT, which is less than one-tenth its current average market price. Operating hours of the plant, fermentable syrup yield and fixed capital are the most significant parameters affecting MSP.
Introduction
Levoglucosan, 1,6-anhydro-β-D-glucopyranose, is the primary product of thermal deconstruction of pure cellulose. It has several properties that make it an attractive chemical building block.1 These properties include chirality, hydroxyl groups at the C-1 anomeric center position and the primary hydroxyl group at C-6, which are protected internally, in addition to the 1,6-anhydro bridge that can be opened during any reaction stage under mild acidic conditions1,2 to produce fragments with the correct relative and absolute configuration and functionality. This allows them to be incorporated into chemical synthesis schemes. However, levoglucosan is currently expensive to synthesize, limiting commercial opportunities for its use.3Levoglucosan is conventionally synthesized from D-glucose by attaching the 6-OH group to the anomeric center to form a second ring structure.4 The commonly used synthesis route consists of a series of tedious, time consuming and expensive steps involving the protection of (reactive) hydroxyl groups, activation of the anomeric center in the saccharide, and subsequent removal of the protecting groups.5 Without the use of protecting hydroxyl groups, it is nearly impossible to convert glucose into a 1,6 anhydrosugar derivative without damaging the inner glycosidic bond.5 As a result of these complexities, pure levoglucosan is expensive to synthesize by conventional means, resulting in prices ranging from $10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per MT.6 This limits its use as a chemical building block for commercially important applications, including the manufacture of plastics, surfactants, explosives, propellants, resins, biodegradable polymers, antiviral agents, and other chiral bioactive natural products.7,8
000 per MT.6 This limits its use as a chemical building block for commercially important applications, including the manufacture of plastics, surfactants, explosives, propellants, resins, biodegradable polymers, antiviral agents, and other chiral bioactive natural products.7,8
An alternative pathway for its production exploits the fact that levoglucosan is the major product of the thermal deconstruction of cellulose,9 the most abundant polymer found in nature, occurring primarily as part of lignocellulosic biomass.1 Fast pyrolysis of inexpensive sources of lignocellulosic biomass such as waste wood or crop residues has the potential to produce large quantities of levoglucosan at commercially attractive prices. Two technical challenges, however, must be overcome to advance this opportunity. The first is the presence of alkali and alkaline earth metals (AAEM) in lignocellulosic biomass, which catalyze the fragmentation of pyranose and furanose rings in polysaccharides, shifting selectivity from anhydrosugars to light oxygenated compounds.10 Although washing AAEM from biomass prior to pyrolysis is unlikely to be cost-effective, a simple infusion of dilute mineral acid has been shown to passivate the catalytic activity of AAEM, allowing anhydrosugar yields from both herbaceous and woody biomass to approach those from pure polysaccharides.11
The second challenge is separation and purification of levoglucosan from other products of fast pyrolysis of biomass. Pyrolysis is premised on the deconstruction of biopolymers to molecules small enough to volatilize and escape the reactor in a stream of inert gas followed by condensation to liquids. Although sugars are generally non-volatile, anhydrosugars and xylose from thermal depolymerization of polysaccharides are sufficiently volatile at pyrolysis temperatures (400–600 °C) to vaporize. However, this is also true of light oxygenated compounds (furans, carboxylic acids, aldehydes and ketones) and phenolic compounds that are co-products of the thermal deconstruction of lignocellulosic biomass. Conventionally, these volatile products are condensed together from the pyrolysis stream as a viscous, acidic emulsion containing hundreds of organic compounds known as bio-oil.12 It is difficult to separate and purify levoglucosan from bio-oil13 due to its complex composition. Typical methods of bio-oil separation and purification include liquid chromatography, solvent extraction, centrifugation, and distillation leading to high cost and difficult scale up.14,15
To overcome problems arising from the condensation of pyrolysis vapors into a single liquid mixture, Pollard et al.16 developed a bio-oil recovery system that separates condensed liquids into fractions with distinct chemical and physical properties.15,16 This is accomplished by carefully controlling coolant temperatures for condensers and electrostatic precipitators used in the bio-oil recovery system. Anhydrosugars and sugars are recovered along with phenolic compounds as high boiling point “heavy ends”. The heavy ends of bio-oil contain very little moisture with most of the water carried to the low boiling point “light ends”. Rover et al.17 have demonstrated that a sugar-rich solution can be recovered from the heavy ends through liquid–liquid extraction using water as the solvent. Stanford et al.18 have successfully removed organic contaminants from this sugar solution using an adsorption resin, SP207, which opens the way to separating and purifying levoglucosan crystals from other pyrolytic sugars.
Techno-economic analysis is a useful tool to evaluate the economic benefits and the costs associated with industrial processes.19 Currently, there are no published studies on the economic feasibility of producing crystallized levoglucosan from fast pyrolysis although previous techno-economic studies on other products of fast pyrolysis using the fractionating condenser system offer a starting point for such an evaluation.20,21
The objective of this research is to evaluate the feasibility of separating, purifying, and crystallizing levoglucosan from bio-oil produced through pyrolysis of lignocellulosic biomass. The process was developed through bench-top experiments with bio-oil produced from a pilot-scale pyrolysis plant. This experimental data was the basis for a discounted cash flow rate of return analysis on the proposed pyrolysis pathway to levoglucosan production.
Experimental and analytical methodologies
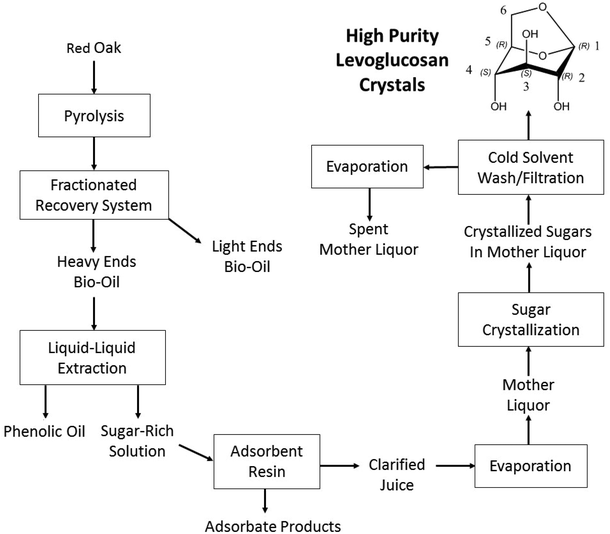
Fig. 1 summerizes the methodology for producing and purifying levoglucosan from lignocellulosic biomass. Red oak was pyrolyzed at 500 °C and the vapor products recovered as two major streams: heavy ends and light ends. The heavy ends contained both water-soluble pyrolytic sugars and largely water-insoluble phenolic compounds. The light ends, consisting of an aqueous phase of carbohydrate degradation products, were not used in this study. Liquid–liquid extraction, using water as the solvent, separated the heavy ends into a sugar-rich solution and phenolic oil. The sugar-rich solution, referred to as juice, was contaminated by partially water-soluble phenolic monomers and light oxygenated compounds derived from the decomposition of the carbohydrate fraction of the biomass. The juice was passed through a column of Sepabeads SP207, which efficiently removed these contaminants, as detailed by Stanford et al.18 The contaminants contain the water-soluble phenolic monomers and the light oxygenates which are referred to as adsorbate products. The clarified juice was rotary evaporated to remove water, resulting in a mother liquor that readily crystallized to a mass of mixed sugar crystals. The crystal mass was washed with cold solvent during filtration, which left behind pure levoglucosan crystals. The levoglucosan that dissolved during the cold methanol wash and the other sugars that went through the filter were rotary evaporated to remove the methanol. The remaining filtrate is denoted as spent mother liquor.Pyrolysis of lignocellulose biomass
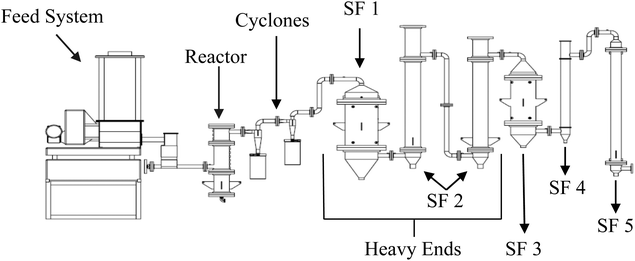
Red oak (Quercus rubra) with moisture content of approximately 10 wt% was procured from Wood Residual Solutions of Montello, WS. Although acid pretreatment prior to pyrolysis could have enhanced levoglucosan yields,11 it was found that this debarked wood produced sufficient levoglucosan to make pretreatment unnecessary for evaluating the feasibility of recovering levoglucosan crystals from bio-oil. The as-received wood was passed through a 60 hp hammer mill with a 3 mm screen, resulting in a particle size range of approximately 0.20 mm to 3.0 mm. A fluidized bed pyrolysis pilot plant, illustrated in Fig. 2, was used to produce the sugar-rich heavy ends used in these experiments. The reactor, fluidized with nitrogen and operated at 500 °C, processed 7.78 kg h−1 of wood to produce bio-oil, non-condensable gases and biochar. Biochar was removed from the gas flow exiting the pyrolyzer using two gas cyclones in series. The bio-oil was collected as stage fractions (SF) according to boiling point. Heavy ends, containing mostly sugars and phenolic compounds, were collected in a water-cooled condenser (SF 1) followed by an electrostatic precipitator (SF 2) operated at 85 °C and 102 °C, respectively. Heavy ends from stage SF 1 and 2 were combined for recovery and purification of levoglucosan. Stages 3–5 collected lower molecular weight fractions of bio-oil, known as light ends, which were not used in this study. Further details on the fluidized bed pyrolyzer and bio-oil recovery system are found in the literature.15,16A sample of the red oak biomass was sent to Celignis Analytical (Limerick, Ireland) for analysis. Total sugars, glucan, xylan, mannan, arbinan, galactan, Klason lignin, acid soluble lignin, extractives, starch and ash were determined. Two replicates were performed for each analysis with averages and standard deviation determined.
Liquid–liquid extraction of heavy ends
Water-soluble sugars in the heavy ends were separated from water-insoluble phenolic compounds by liquid–liquid extraction utilizing water as the solvent. Briefly, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio of water to heavy ends was mechanically stirred with a drill press equipped with a stainless-steel open paddle for 10 to 15 min at 23 °C. The samples were placed on a shaker table (MaxQ 2506, Thermo Scientific®, HanoverPark, IL) for 30 min at 250 motions per min and centrifuged (accuSpin 1R, Thermo Scientific®, Hanover Park, IL) at 2561g force for 30 min. The mixture was decanted to recover the water-soluble fraction of the heavy ends. The remaining water-insoluble fraction of the heavy ends, referred to as phenolic oil, was not used in this study. A detailed description of this extraction process with optimized liquid–liquid extraction conditions can be found in literature.17
1 (w/w) ratio of water to heavy ends was mechanically stirred with a drill press equipped with a stainless-steel open paddle for 10 to 15 min at 23 °C. The samples were placed on a shaker table (MaxQ 2506, Thermo Scientific®, HanoverPark, IL) for 30 min at 250 motions per min and centrifuged (accuSpin 1R, Thermo Scientific®, Hanover Park, IL) at 2561g force for 30 min. The mixture was decanted to recover the water-soluble fraction of the heavy ends. The remaining water-insoluble fraction of the heavy ends, referred to as phenolic oil, was not used in this study. A detailed description of this extraction process with optimized liquid–liquid extraction conditions can be found in literature.17
Purification and recovery of levoglucosan
The sugar rich solution was passed through a wet-packed column of Sepabeads SP207 (Sigma Aldrich, St Louis, Missouri) at a steady rate of 2 column volumes per h (16.6 ml min−1). Stanford et al.18 provides a detailed description of the operation and performance of this filtration process. The product from the filtration is referred to as clarified juice in analogy with the name used by the sugar refining industry to describe the sugar-rich solution pressed from sugar cane or sugar beets. A rotary evaporator (Hei-VAP Precision, Heidolph, Schwabach, Germany) operated at 72 mbar with a bath temperature of 40 °C and coolant temperature of −10 °C for 6 hours, evaporated the clarified juice to a concentrated sugar solution referred to as mother liquor. As a result of filtration, the mother liquor was sufficiently pure for crystals of mixed sugars to readily form when cooled to 4 °C. The mixture of crystal mass and mother liquor were rinsed with cold methanol (−20.2 °C) and vacuum filtered using a Büchner funnel with 42 Whatman® filter paper, which left behind almost pure levoglucosan crystals on the filter paper. The mother liquor from cold solvent washing (spent mother liquor) contained significant dissolved levoglucosan and other kinds of sugars produced from pyrolysis of lignocellulose. The pure levoglucosan crystals were dried in an Isotemp vacuum oven Model 282A (Fisher Scientific/Thermo Scientific, Hanover Park, IL) at 40 °C using 5 inches of mercury.Analysis of heavy ends
Ultimate analysis of the heavy ends of the bio-oil was performed with an Elementar® elemental analyzer (vario MICRO cube) (Elementar Americas Inc, Ronkonkoma, NY). The approximately 5 mg samples were combusted at 900 °C and the combustion products (carbon dioxide, water, and nitric oxide) were characterized by a thermal conductivity detector. Weight percentages of the C, H, and N were characterized by a thermal conductivity detector and were calculated based on the amount of each combustion product. The samples were inserted into the combustion chamber for analysis with a minimum of three trials performed for each analysis with averages and standard deviation determined.22,23Sugar analyses
Sugar analysis of the mother liquor prior to crystallization and the spent mother liquor (after the cold solvent wash and rotary evaporation of the methanol) were performed using high performance liquid chromatography (HPLC) equipped with a refractive index (RI) detector and two Bio-Rad Aminex HPX-87P with a guard column. The flow rate was 0.6 mL min−1 18.2 Ω distilled water with the column temperature 75 °C. The RI detector was calibrated for cellobiosan, levoglucosan, sorbitol, xylose, mannose, and galactose. All standards were obtained from Carbosynth (cellobiosan) and Fisher Scientific (xylose, mannose, sorbitol, galactose, levoglucosan). Approximately 0.5 g of the mother liquor prior to crystallization or spend mother liquor after the cold solvent wash and rotary evaporation were dissolved in 5 mL deionized water and mixed. Purity of crystallized levoglucosan was determine by dissolving the crystals in deionized water to a concentration of 0.5 to 2.0 mg mL−1. Nine trials were performed with standard deviation and 99% confidence interval determination. All solutions were filtered through a Whatman® 0.45 micron glass microfiber filter and 25 μL were injected on the HPLC.22GC/FID fitted with a PolyArc (Activated Research Company, Eden Prairie, MN) was used to quantify volatiles in the spent mother liquor after the cold solvent wash and rotary evaporation of the methanol. The GC/FID (Bruker Daltonics, Inc. Fremont, CA) was equipped with a flame ionization detector and fitted with a Zebron ZB-1701 (60 m × 0.25 mm × 0.25 μm film thickness) capillary column (Phenomenex, Torrance, CA). The operating system was a Galaxie Chromatography Data System (version 1.9.302.530) from Bruker Daltonics (Bruker Corporation, Fremont, CA). Helium (99.9995%) was the carrier gas with a constant flow rate of 1.0 mL min−1. Helium make-up was 25 mL min−1, hydrogen flow was at 30 mL with an air flow of 300 mL min−1. The GC oven was programmed to hold for 3 min at 35 °C, ramped at 5 °C min−1 to 280 °C, and held for 4 min for a total of 56.0 min. A sample volume of 1 μL was injected using a Varian CP 8400 (Bruker Daltonics, Inc., Fremont, CA) auto sampler with a split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The PolyArc, which enables quantification of chemical compounds without calibration standards, was used to analyze the spent mother liquor after the cold solvent wash and rotary evaporation of the methanol. The internal standards used with the PolyArc were toluene and phenanthrene at 5 wt% each in 95 wt% HPLC grade methanol. The solvent/internal standards solution was mixed with 20 wt% spent mother liquor, which was then analyzed by gas chromatography (GC) (7890B, Agilent Technologies, USA) mass spectroscopy (MS) (5977A, Agilent Technologies, USA) fitted with the same Zebron column and the Bruker 430 GC/FID to identify chemical constituents within the sample. Identification of compounds was accomplished using NIST MS Library Version 2.0. Three trials were performed with averages and standard deviation determined.
20. The PolyArc, which enables quantification of chemical compounds without calibration standards, was used to analyze the spent mother liquor after the cold solvent wash and rotary evaporation of the methanol. The internal standards used with the PolyArc were toluene and phenanthrene at 5 wt% each in 95 wt% HPLC grade methanol. The solvent/internal standards solution was mixed with 20 wt% spent mother liquor, which was then analyzed by gas chromatography (GC) (7890B, Agilent Technologies, USA) mass spectroscopy (MS) (5977A, Agilent Technologies, USA) fitted with the same Zebron column and the Bruker 430 GC/FID to identify chemical constituents within the sample. Identification of compounds was accomplished using NIST MS Library Version 2.0. Three trials were performed with averages and standard deviation determined.
Acid content of the mother liquor (prior to crystallization) was determined by ion-exchange chromatography (IC). Acetic, formic, glycolytic, and propionic acids were quantified using a Dionex ICS3000 (Thermo Scientific®, Sunnyvale, CA) equipped with a conductivity detector and an Anion Micromembrane Suppressor AMMS-ICE300. The regenerate for the suppressor was 5 mM tetrabutylammonia hydroxide (TBAOH) at 4–5 mL min−1 flowrate. The eluent was 1.0 mM heptaflourobutyric acid with an IonPac® ICE-AS1 4 × 50 mm guard column and IonPac® ICE-AS1 4 × 250 mm analytical column. The flow rate was 0.120 mL min−1 at 19 °C. The software was Dionex Chromeleon (Thermo Scientific®, Sunnyvale, CA) version 6.8. Samples of mother liquor (prior to crystallization) were prepared using 6 mL deionized water and 1.5 mL methanol. All samples were filtered with a Whatman® 0.45 μL Glass Microfiber (Thermo Scientific® Hanover Park, IL) syringe filter prior to IC analysis. Samples were analyzed in duplicate with averages and standard deviations determined.24
Techno-economic analysis
The techno-economic analysis assumes a biorefinery processing 250 dry metric tonne per day (MTPD) of red oak via autothermal pyrolysis25 to produce pure levoglucosan crystals as its primary product. The biorefinery also generates water-soluble sugars (spent mother liquor), phenolic oil and biochar as co-products. The plant has operating capacity of 90%, which is equivalent to 7884 hours per year. The process design is based on the experimental data described in this study and previous work by Li et al.26Autothermal pyrolysis uses air at low equivalence ratio to support partial oxidation of pyrolysis products for the purpose of supplying the enthalpy for pyrolysis.25 There are four main advantages of autothermal pyrolysis: firstly, air-blown operation eliminates the need for inert gases to fluidize the bed and removes ancillary heat transfer equipment from the system, which simplify reactor design. Secondly, biomass feed rate is several times higher than for conventional pyrolysis, depending upon reactor size, resulting in significant process intensification. Lastly, capital costs are reduced about 25%. These advantages increase sugar productivity and reduce capital costs in the economic analysis.
Aspen Plus 10™ was employed to build the process model and obtain mass and energy balances for levoglucosan crystals production from red oak. It includes unit operations for biomass preparation including drying and grinding; autothermal pyrolysis; product fractionation and recovery; and sugar crystallization and purification. Both the mass and energy balance of each unit operation and operating conditions were employed to select and size process equipment in the analysis, while cost of equipment was estimated using Aspen Process Economic Analyzer (APEA). Additionally, project installation factors were obtained from Peters et al.27
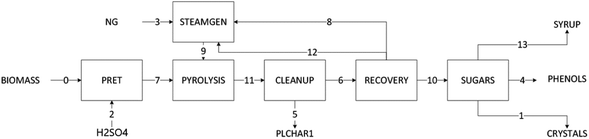
Fig. 3 shows a block diagram of the process design with the key material and energy streams tabulated in Table 1. Red oak at 50 wt% moisture content and >10 mm average particle diameter is pretreated prior to pyrolysis, which includes grinding to particles of approximately 3 mm size, and infusion of 0.4 wt% sulfuric acid solution into the biomass at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio to passivate inorganic materials in the biomass for the purpose of enhancing sugar production during pyrolysis.28 for the purpose of enhancing sugar production during pyrolysis. The biomass is then dried to a moisture content below 10 wt%. The biomass is dried with heat from burning tail gas (light ends vapor and non-condensable gases) from the pyrolysis process. The pretreated and dried biomass feeds into the pyrolysis section with 7 wt% moisture content and 3 mm particle diameter. To attain autothermal processing of biomass, the pyrolysis reactor is fluidized with air at an equivalence ratio of 0.1, temperature of 500 °C, and atmospheric pressure.25 Other than the autothermal reactor, most of the design of the pyrolysis plant is based on work by the National Renewable Energy Laboratory (NREL). The NREL design represents a 2000 metric tonne per day fluidized bed reactor. For this study, plant size was scaled down to 250 metric tonne per day of capacity while the autothermal reactor was assumed to process five times more feedstock than a similar-sized conventional pyrolysis reactor.25
1 mass ratio to passivate inorganic materials in the biomass for the purpose of enhancing sugar production during pyrolysis.28 for the purpose of enhancing sugar production during pyrolysis. The biomass is then dried to a moisture content below 10 wt%. The biomass is dried with heat from burning tail gas (light ends vapor and non-condensable gases) from the pyrolysis process. The pretreated and dried biomass feeds into the pyrolysis section with 7 wt% moisture content and 3 mm particle diameter. To attain autothermal processing of biomass, the pyrolysis reactor is fluidized with air at an equivalence ratio of 0.1, temperature of 500 °C, and atmospheric pressure.25 Other than the autothermal reactor, most of the design of the pyrolysis plant is based on work by the National Renewable Energy Laboratory (NREL). The NREL design represents a 2000 metric tonne per day fluidized bed reactor. For this study, plant size was scaled down to 250 metric tonne per day of capacity while the autothermal reactor was assumed to process five times more feedstock than a similar-sized conventional pyrolysis reactor.25
| ID | Name | Description | Temperature (°C) | Pressure (Pa) | Mass flow (tonne per day) |
|---|---|---|---|---|---|
| 0 | BIOMASS | Biomass feedstock (50% moisture) | 25 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
500 |
| 1 | CRYSTALS | Crystalized levoglucosan product | 25 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
6.6 |
| 2 | H2SO4 | Sulfuric acid reactant | 25 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
8.0 |
| 3 | NG | Natural gas for drying | 40 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
0.0 |
| 4 | PHENOLS | Phenolic oil product | 25 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
80.9 |
| 5 | PLCHAR1 | Biochar product | 25 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
32.7 |
| 6 | PLCLGAS | Clean pyrolysis vapors without solids | 500 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
239 |
| 7 | PLFEED3M | Biomass feedstocks (3 mm-diameter) sized | 101 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
272 |
| 8 | PLNCG | Pyrolysis non-condensable gases | 18 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
54.0 |
| 9 | PLRECYCL | Pyrolysis gas recycle stream | 200 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
317 |
| 10 | PLSF12 | Heavy and light ends | 103 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
155 |
| 11 | PLVAPORS | Pyrolysis vapors (with solids) | 500 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
272 |
| 12 | SF3–5 | Pyrolysis tail gas | 120 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
28.6 |
| 13 | SYRUP | Spent mother liquor product | 25 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325 |
39.8 |
Pyrolysis vapors exiting the reactor progress through two gas cyclones in series to remove biochar, which is sold as co-product. The cyclones collectively recover up to 99% of solids from the gas flow. Pyrolysis vapors enter the bio-oil recovery section, which fractionates and condenses the vapors into heavy ends and light ends. The heavy ends feed into the sugar recovery section that includes liquid–liquid extraction of sugars; resin removal of phenolic contaminants from the sugar solution; and crystallization and methanol rinsing. The final products from the heavy ends include levoglucosan crystals, fermentable syrup (spent mother liquor), and phenolic oil.
Liquid–liquid extraction contacts the heavy ends with water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio followed by separation into a water-soluble fraction (sugar-rich solution) and water-insoluble fraction (phenolic oil). The sugar-rich solution goes to a resin purification unit to remove contaminants followed by an evaporation/crystallization unit (modeled as a yield reactor) where crystals of mixed sugars are obtained. The design specification for this analysis is 2.75 wt% levoglucosan crystal yield from red oak, which is based on the experimental results described in this paper. Finally, 99% of the crystals are recovered as a pure stream in a separator unit. The remaining water-soluble sugars leave as spent mother liquor, which is sold as fermentable syrup.
1 ratio followed by separation into a water-soluble fraction (sugar-rich solution) and water-insoluble fraction (phenolic oil). The sugar-rich solution goes to a resin purification unit to remove contaminants followed by an evaporation/crystallization unit (modeled as a yield reactor) where crystals of mixed sugars are obtained. The design specification for this analysis is 2.75 wt% levoglucosan crystal yield from red oak, which is based on the experimental results described in this paper. Finally, 99% of the crystals are recovered as a pure stream in a separator unit. The remaining water-soluble sugars leave as spent mother liquor, which is sold as fermentable syrup.
The economic feasibility of this process is evaluated using a multi-year discounted cash flow rate of return (DCFROR) analysis. A financial spreadsheet that accounts for the biorefinery plant life of 10 years, 8% loan interest rate and a 10-year payback period and other financial assumptions was used to compute the minimum levoglucosan crystals selling price (MSP). The financial assumptions are tabulated in Table 2. All costs are reported in 2015$ basis.
| Parameter | Assumptions |
|---|---|
| Equity | 40% |
| Plant life | 10 years |
| Construction period | 1 year |
| Depreciation period | 7 years, 200 DDB |
| Working capital | 5% of FCI |
| Plant salvage value | 0 |
| Revenue & cost during startup (% of normal) | Revenue: 50% |
| Variable costs: 75% | |
| Fixed costs: 100% | |
| Interest rate for financing | 8% annually |
| Internal rate of return | 10% |
The annual fixed operating cost of the biorefinery consists of cost of labor, overhead, maintenance and insurance. Overhead costs are 90% of the cost of labor, cost for maintenance is 3% of Inside Battery Limits (ISBL) and insurance is 0.7% of the Fixed Capital Investment. Labor cost is based on a modified analysis of a previous NREL study29 and is based on the number of employees and their salary rates. The breakdown of the labor cost is tabulated in Table 3.
| Position | Salary ($ per year) | Number of employees |
|---|---|---|
| Maintenance supervisor | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1 |
| Maintenance technician | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
8 |
| Lab manager | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1 |
| Lab technician | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1 |
| Shift supervisor | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
5 |
| Shift operators | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
5 |
| Yard employees | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2 |
Variable operating cost assumptions are listed in Table 4. These include feedstock cost, process water, utility costs, and by-product credits. Previous studies have reported that feedstock cost at the plant gate vary from $30–$60 per MT.30,31 In this study, feedstock cost is assumed to be $41 per MT, which is comparable and within the range of previous studies. The cost of solids handling is assumed to be $8 per MT as suggested in a recent NREL study.32 Additionally, the fermentable syrup credit of $406 per MT assumed in this study is based on a previous economic analysis of cellulosic ethanol from lignocellulosic biomass and adjusted for inflation.34 Both phenolic oils and biochar are valued at $50 per MT, which assumes no valorization beyond their use as boiler fuel. Based on previous studies, the cost of electricity is reported to vary from $0.06–$0.23 kW−1 h−1.33,34 We assumed the cost of electricity to be 6.7′ kW−1 h−1. Lastly, the methanol used to recover levoglucosan crystals has a market cost ranging between $310 and $850 per MT.35 This study assumes a cost of $500 per MT. The methanol recovery rate is assumed to be 99%.36 We estimate the methanol recovery cost to be $7 per MT of methanol based on work by Shahandeh et al.37 This cost includes the annualized capital and operating costs of the distillation process.
| Parameter | Flows (daily) | Price |
|---|---|---|
| Red oak (dry) | 250 MT | $41 per MT |
| Solids handling | 50 MT | $8 per MT |
| Process water | 95 MT | $0.2 per MT |
| Methanol | 0.285 MT | $500 per MT |
| Electricity | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MJ (4990 kWh) 000 MJ (4990 kWh) |
$0.0186 per MJ ($0.067 kW−1 h−1) |
| Co-products | ||
| Syrup (dry basis) | 39.8 MT | −$406 per MT |
| Phenolic oil | 80.9 MT | −$50 per MT |
| Biochar (ash-free) | 32.7 MT | −$50 per MT |
Results and discussion
Biomass composition
The red oak biomass composition is shown in Table 5. The red oak biomass contained 40% (dry matter biomass) of glucan (C6 sugar). The hemicellulose contributes toward C6 sugars in the form of glucan, mannan and galactan. The quantified C5 sugars from hemicellulose are arabinan and xylan. The major part of the lignin is Klason lignin (20.3% dry matter basis) while the acid soluble lignin is only 2.95% (dry matter basis). Other components include extractives and ash.| Red oak lignocellulosic component | Dry matter (%) |
|---|---|
| Total sugars | 58.6 ± 0.43 |
| Glucan | 40.0 ± 0.22 |
| Xylan | 15.7 ± 0.23 |
| Mannan | 1.30 ± 0.34 |
| Arabinan | 0.34 ± 0.01 |
| Galactan | 0.92 ± 0.01 |
| Klason lignin | 20.3 ± 0.33 |
| Acid soluble lignin | 2.95 ± 0.03 |
| Extractives | 6.85 ± 0.07 |
| Ash | 0.40 ± 0.07 |
| Total | 89.1 |
Yields of pyrolysis products
The yield of heavy ends (combined SF1 and SF2) from red oak pyrolysis was 25.5 wt% while the yield of light ends (combined SF3–5) was 31.5 wt% for a total bio-oil yield of 57.0 wt%. Yields of char and non-condensable gases were 14.0 wt% and 21.1 wt%, respectively, resulting in an overall mass closure of 92.1 wt%.Water extraction of the heavy ends produced a sugar-rich, water-soluble fraction and a phenolic-rich, water insoluble fraction. The yields of these products on a moisture-free basis and their carbon contents are given in Table 6. The yield of water-soluble fraction from the heavy ends was 48.1 wt% and contained 48.5 wt% carbon. The yield of sugar from water extraction of heavy ends was 10.9 wt% and consisted mostly of levoglucosan (7.47 wt%) with smaller amounts of cellobiosan, xylose, galactose, and mannose. The yield of the water-insoluble fraction was 51.9 wt% and contained 72.6 wt% carbon.
| Yield (wt%) | |
|---|---|
| Water-soluble fraction | 48.1 |
| Levoglucosan | 7.47 ± 0.033 |
| Cellobiosan | 1.25 ± 0.327 |
| Xylose | 1.34 ± 0.053 |
| Galactose | 0.725 ± 0.011 |
| Mannose | 0.105 ± 0.001 |
| Total sugar | 10.89 ± 0.123 |
| Water-insoluble fraction | 51.9 |
| Carbon content of water extracted fractions of heavy ends | Carbon content (wt%) |
|---|---|
| Water-soluble fraction | 48.5 ± 0.313 |
| Water-insoluble fraction | 72.6 ± 0.158 |
Crystalized levoglucosan recovery
The sugar-rich, water-soluble fraction produced from liquid–liquid extraction of the heavy ends was eluted through the Sepabeads SP207 resin. After evaporation of water, the resulting mother liquor was sufficiently pure for crystallization to occur when cooled to 4 °C. The ease in which crystals were produced suggests that crystallization techniques commonly employed in industry could be used. Typically, industrial crystallization processes involve numerous steps prior to obtaining the final product, comprised of seeding, cooling, and filtration.38The compositions of the mother liquor before and after recovery of crystalized levoglucosan are shown in Table 7. Total sugar content of the mother liquor prior to crystallization was 81.2 wt% db. Levoglucosan was the most abundant sugar at 44.7 wt% db of the mother liquor before crystallization followed by mannose and cellobiosan at 12.8 and 11.7 wt% db, respectively. Xylose and galactose were present at 9.31 wt% db and 2.72 wt% db, respectively.
| Constituent | Mother liquor before crystallization | Mother liquor after crystallization |
|---|---|---|
| wt% (db) | wt% (db) | |
| Levoglucosan | 44.7 ± 0.044 | 33.7 ± 0.078 |
| Mannose | 12.8 ± 0.064 | 16.3 ± 0.103 |
| Cellobiosan | 11.7 ± 0.060 | 16.5 ± 0.060 |
| Xylose | 9.31 ± 0.019 | 11.8 ± 0.064 |
| Galactose | 2.72 ± 0.004 | 2.99 ± 0.043 |
| Total sugar content | 81.2 ± 0.038 | 81.3 ± 0.070 |
| Other compounds (by difference) | 18.8 | 19.2 ± 0.100 |
| Total | 100 | 100 |
After removal of crystals, levoglucosan concentration in the spent mother liquor was 33.7 wt% db. Thus, only 24.8% of the levoglucosan in the mother liquor was recovered by the one-stage crystallization process employed in this laboratory study. Multi-stage crystallization, as regularly practiced in industry, is expected to significantly increase the recovery of crystallized levoglucosan although it is difficult to quantify. The purity of these levoglucosan crystals was 102.5% ± 3.109% at the 99% confidence level. Purity likely could be increased to over 99.99% by utilizing resin columns in series versus one pass through the resin employed in this study.
Cellobiosan, xylose, galactose, and mannose increased in the spent mother liquor, as expected, but slightly more than anticipated. This is thought to be attributed to the loss of volatiles during the repeated handling of the sample throughout crystal removal and rotary evaporation of the solvent.
Volatile compounds other than anhydrosugars in the spent mother liquor were quantified by GC/PolyArc/FID and ranged from 4.45–4.60 wt% db. Carboxylic acid content was 1.71 wt% db and consisted of glycolic and formic acids. These relatively low concentrations is attributed to the unique bio-oil collection system, which collected most of the light oxygenated compounds (carboxylic acids, ketones, aldehydes, alcohols) in condensers downstream of the point where heavy ends were collected.
Technoeconomic analysis
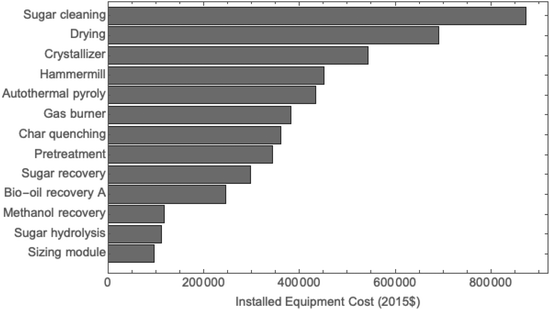
The total project investment of this plant is estimated as $18 MM. The full list of equipment and their cost are shown in Fig. 4. Cost for sugar cleaning is the most expensive, followed by the biomass pretreatment drying unit and crystallizer, which assumes 5 hours residence time to complete crystallization. Since the light ends are not condensed but rather burned with the non-condensable gas as tail gas to provide process heat, no wastewater treatment facility was needed. The facility includes a distillation tower for recovering methanol. The feed composition consists of phenols and organic species (0.4 wt%), water (3 wt%), and methanol. The distillate composition was 99.7% pure methanol. Most of the remaining units are standard engineering equipment such as heat exchangers and burners. Cost of the autothermal pyrolysis reactor is estimated to be in the order of $430![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
As detailed in Table 1 of the Methodologies section, the plant yields 6.6 MTPD of levoglucosan crystals. Co-products include 80.9 MTPD of phenolic oil, 39.8 MTPD of fermentable syrup, and 32.7 MTPD of biochar, which provide annual operating credits of $1.33 MM, $5.29 MM, and $0.538 MM. Annual net operating cost for the plant is $2.88 MM. The fixed operating costs, which include cost for insurance, maintenance and overhead, is $2.44 MM; labor cost is $0.93 MM; and variable operating costs is $3.66 MM on an annual basis.
The contributions of various operating costs and credits to the MSP are summarized in Fig. 5. Gross operating cost is $4500 per MT of levoglucosan crystals. Feedstock dominates operating costs, representing 33% of the gross operating cost. Fermentable syrup, phenolic oil and biochar provide credits equal to 70% of the gross operating costs. These credits reduce the minimum selling price (MSP) of levoglucosan from $4500 per MT to $1333 per MT. This price is much lower than the current market price for levoglucosan crystals synthesized from glucose, which ranges between $10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per MT.6
000 per MT.6
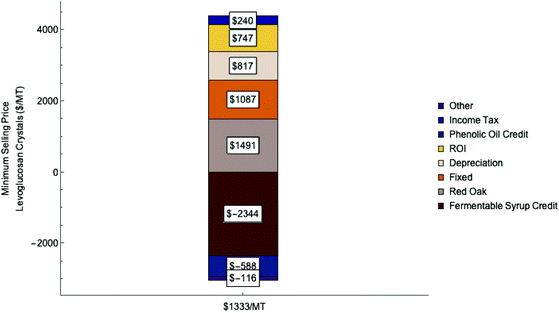 | ||
| Fig. 5 Operating costs ($ per MT of levoglucosan) in the production of crystalline levoglucosan from lignocellulosic biomass. | ||
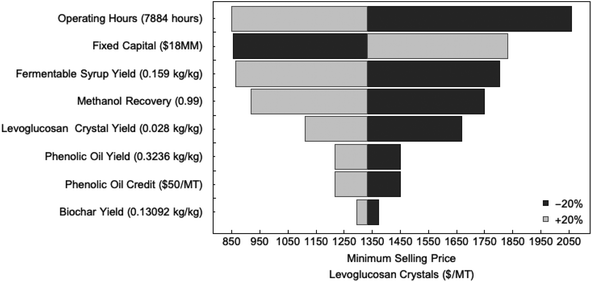
Sensitivity analysis was also conducted to determine the most significant operating parameters on the MSP. The results of the analysis are illustrated in Fig. 6. Operating hours, followed by fermentable syrup yield and fixed capital are the three most significant parameters affecting the MSP, while phenolic oil yield, phenolic oil credit and biochar yield affect the MSP of anhydrosugar crystals the least. Increasing fermentable syrup yield or operating cost by 20% can reduce MSP by 37%, while a 20% decrease in fixed capital from $18 MM to $14 MM can reduce MSP by 37%. Improving methanol recovery by 20% would reduce MSP by 27%. On the other hand, a 20% increase in yields of either phenolic oil or biochar will only reduce the MSP by 8% and 4%, respectively.
Conclusions
We have demonstrated the production of crystalized levoglucosan through the fast pyrolysis of lignocellulosic biomass. A combination of fractionating bio-oil recovery, liquid–liquid extraction, and resin filtration yielded a mother liquor containing 81.2 wt% db total sugars of which 44.7 wt% db was levoglucosan. One pass filtration with Sepabeads SP207 sufficiently removed phenolic compounds and other contaminants to readily crystallize sugars from the mother liquor. Solvent washing of the crystal mass allowed 24.7% recovery of levoglucosan from the mother liquor as crystals, which showed purity of 102.5% ± 3.109% wt% db at the 99% confidence level. Techno-economic analysis indicates that levoglucosan crystals could be produced via this process at a cost of $1333 per MT, which is ten times lower than the current market price range. Sensitivity analysis demonstrated that increases in syrup yield and operating hours and decreases in fixed capital costs have the best prospects for further reducing the cost of levoglucosan. This study offers a low-cost pathway for production of levoglucosan as a platform chemical in the production of biobased products.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors greatly acknowledge funding support from the Department of Energy/Rapid Advancement in Process Intensification Deployment (DOE/RAPID) Institute. The authors also extend their gratitude to Lysle Whitmer, Andrew Friend, and Jordan Funkhouser for the operation of the pyrolysis reactor. We thank Patrick Johnston for PolyArc guidance and Patrick Hall for preparing the mother liquor in addition to performing HPLC and IC analysis. Undergraduate students Clint Jochems and Rachel Korntved and graduate student John Hoyt are acknowledged for their help with chemical analyses.References
- K. Krohn, D. Gehle and U. Flörke, Eur. J. Org. Chem., 2005, 2841–2848 CrossRef CAS.
- P. J. Hodges and G. Procter, Tetrahedron Lett., 1985, 26, 4111–4114 CrossRef CAS.
- L. Chen, J. Zhao, S. Pradhan, B. E. Brinson, G. E. Scuseria, Z. C. Zhang and M. S. Wong, Green Chem., 2016, 18, 5438–5447 RSC.
- K. C. Marion, Z. Wooke and N. L. B. Pohl, Carbohydr. Res., 2018, 468, 23–29 CrossRef CAS.
- T. Tanaka, W. C. Huang, M. Noguchi, A. Kobayashi and S. Shoda, Tetrahedron Lett., 2009, 50, 2154–2157 CrossRef CAS.
- Alibaba.com, Price range estimated from offerings posted on Alibab.com.
- C. M. Lakshmanan, B. Gal-or and H. E. Hoelscher, Prod. Res. Dev., 1969, 8, 261–267 CrossRef CAS.
- V. Bailliez, A. Olesker and J. Cleophax, Tetrahedron, 2004, 60, 1079–1085 CrossRef CAS.
- P. R. Patwardhan, J. A. Satrio, R. C. Brown and B. H. Shanks, J. Anal. Appl. Pyrolysis, 2009, 86, 323–330 CrossRef CAS.
- P. R. Patwardhan, J. A. Satrio, R. C. Brown and B. H. Shanks, Bioresour. Technol., 2010, 101, 4646–4655 CrossRef CAS PubMed.
- N. Kuzhiyil, D. Dalluge, X. Bai, K. H. Kim and R. C. Brown, ChemSusChem, 2012, 5, 2228–2236 CrossRef CAS PubMed.
- R. C. Brown and T. R. Brown, Biorenewable Resources: Engineering New Products from Agriculture, Wiley Blackwell, Ames, IA, 2nd edn, 2014 Search PubMed.
- K. Meile and A. Zhurinsh, Key Eng. Mater., 2017, 721–721, 82–86 Search PubMed.
- S. Wang, Y. Gu, Q. Liu, Y. Yao, Z. Guo, Z. Luo and K. Cen, Fuel Process. Technol., 2009, 90, 738–745 CrossRef CAS.
- M. R. Rover, P. A. Johnston, L. E. Whitmer, R. G. Smith and R. C. Brown, J. Anal. Appl. Pyrolysis, 2014, 105, 262–268 CrossRef CAS.
- A. S. Pollard, M. R. Rover and R. C. Brown, J. Anal. Appl. Pyrolysis, 2012, 93, 129–138 CrossRef CAS.
- M. R. Rover, P. A. Johnston, T. Jin, R. G. Smith, R. C. Brown and L. Jarboe, ChemSusChem, 2014, 7, 1662–1668 CrossRef CAS PubMed.
- J. P. Stanford, P. H. Hall, M. R. Rover, R. G. Smith and R. C. Brown, Sep. Purif. Technol., 2018, 194, 170–180 CrossRef CAS.
- L. Lin, A. Shah, H. Keener and Y. Li, Waste Manage., 2019, 85, 405–416 CrossRef CAS PubMed.
- W. Li, Q. Dang, R. C. Brown, D. Laird and M. M. Wright, Bioresour. Technol., 2017, 241, 959–968 CrossRef CAS.
- B. Li, L. Ou, Q. Dang, P. Meyer, S. Jones, R. Brown and M. Wright, Bioresour. Technol., 2015, 196, 49–56 CrossRef CAS.
- F. Y. S. Choi, P. A. Johnston, R. C. Brown, B. H. Shanks and K.-H. Lee, J. Anal. Appl. Pyrolysis, 2015, 112, 401–402 CrossRef CAS.
- M. R. Rover, P. H. Hall, P. A. Johnston, R. G. Smith and R. C. Brown, Fuel, 2015, 153, 224–230 CrossRef CAS.
- M. R. Rover, P. A. Johnston, B. P. Lamsal and R. C. Brown, J. Anal. Appl. Pyrolysis, 2013, 104, 194–201 CrossRef CAS.
- J. P. Polin, C. A. Peterson, L. E. Whitmer, R. G. Smith and R. C. Brown, Appl. Energy, 2019, 276–285 CrossRef CAS.
- W. Li, Q. Dang, R. Smith, R. C. Brown and M. M. Wright, ACS Sustainable Chem. Eng., 2017, 5, 1528–1537 CrossRef CAS.
- R. E. Peters, M. S. Timmerhaus and K. D. West, Plant Design and Economics for Chemical Engineers. Plant Design and Economics for Chemical Engineers, McGraw-Hill, 2004 Search PubMed.
- D. L. Dalluge, T. Daugaard, P. Johnston, N. Kuzhiyil, M. M. Wright and R. C. Brown, Green Chem., 2014, 16, 4144–4155 RSC.
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof, M. Worley, D. Sexton and D. Dudgeon, Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol: dilute-acid pretreatment and enzymatic hydrolysis of corn stover, Golden, CO, United States, 2011 Search PubMed.
- J. Aden, A. Ruth, M. Ibsen, K. Jechura, J. Neeves, K. Sheehan, J. Wallace, B. Montague, L. Slayton and A. Lukas, Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover, United States, 2002 Search PubMed.
- Y. Zhu and S. B. Jones, Techno-economic Analysis for the Thermochemical Conversion of Lignocellulosic Biomass to Ethanol via Acetic Acid Synthesis, Richland, WA, United States, 2009 Search PubMed.
- A. Dutta, A. Sahir, E. Tan, D. Humbird, L. J. Snowden-Swan, P. A. Meyer, J. Ross, D. Sexton, R. Yap and J. Lukas, Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels: Thermochemical Research Pathways with In Situ and Ex Situ Upgrading of Fast Pyrolysis Vapors, 2015 Search PubMed.
- A. Akbulut, Energy, 2012, 44, 381–390 CrossRef.
- D. Balussou, A. Kleyböcker, R. McKenna, D. Möst and W. Fichtner, Waste Biomass Valorization, 2012, 3, 23–41 CrossRef CAS.
- Alibaba: Chemical Price List, Chemicals Price List Suppliers and Manufactures.
- W. Li, A. Ghosh, D. Bbosa, R. Brown and M. M. Wright, ACS Sustainable Chem. Eng., 2018, 6, 16515–16524 CrossRef CAS.
- H. Shahandeh, M. Jafari, N. Kasiri and J. Ivakpour, Energy, 2015, 80, 496–508 CrossRef.
- O. Velazquez-Camilo, E. Bolaños-Reynoso, E. Rodriguez and J. Alvarez-Ramirez, J. Food Eng., 2010, 100, 77–84 CrossRef.
| This journal is © The Royal Society of Chemistry 2019 |