Eco-efficient synthesis of 2-quinaldic acids from furfural†
Abstract
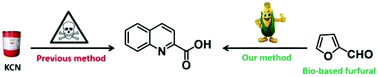
Quinaldic acids are important fine chemicals. Nowadays, industrial methods to synthesize quinaldic acids rely heavily on a three-step process established based on the Reissert reaction, which involves however the use of highly toxic potassium cyanide. In this paper, a novel cyclization of aniline with ethyl 4,4-diethoxycrotonate was realized, which offered ethyl quinaldate in good yield. Based on this reaction, an eco-efficient method to prepare quinaldic acids was developed, which involves the following three steps: (i) synthesis of ethyl 4,4-diethoxycrotonate through photooxidation of furfural and a consecutive ring-opening alcoholysis; (ii) cyclization of ethyl 4,4-diethoxycrotonate with aniline, and (iii) hydrolysis of the generated ethyl quinaldate. This new method not only avoids the use of toxic potassium cyanide but also meets many salient features of green chemistry, such as the use of bio-based feedstocks, environmentally benign metal-free conditions and good reaction yields.
- This article is part of the themed collections: International Symposium on Green Chemistry 2019 and 2019 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...