Confinement of Brønsted acidic ionic liquids into covalent organic frameworks as a catalyst for dehydrative formation of isosorbide from sorbitol†
Abstract
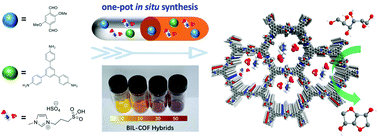
The confinement of Brønsted acidic 1-methyl-3-(3-sulfopropyl)-1H-imidazol-3-ium hydrosulfate ([PSMIm][HSO4]) into the channel walls of two-dimensional (2D) COFs using a one-pot self-assembly strategy was achieved by incorporating an imine-linked TPB–DMTP–COF (TPB, triphenylbenzene; DMTP, dimethoxyterephthaldehyde) as the host. An appropriate loading of [PSMIm][HSO4] is crucial for the BIL–COF hybrids to maintain proper geometry in the channel and sufficient acidic sites for the sorbitol substrate and sorbitan intermediate to enter and react. The best yield of isosorbide (97%) from sorbitol to date was obtained in the presence of BIL–COF-30 as the catalyst under optimized conditions. Besides, BIL–COF-30 can be recycled for at least five runs without activity loss.


 Please wait while we load your content...
Please wait while we load your content...