Open Access Article
Open Access ArticleDirect one-step synthesis of a formally fully bio-based polymer from cellulose and cinnamon flavor†
Daisuke
Hirose
 *,
Samuel Budi Wardhana
Kusuma
,
Daiki
Ina
,
Naoki
Wada
and
Kenji
Takahashi
*,
Samuel Budi Wardhana
Kusuma
,
Daiki
Ina
,
Naoki
Wada
and
Kenji
Takahashi
 *
*
Graduate School of Natural Science and Technology, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan. E-mail: dhirose@se.kanazawa-u.ac.jp; ktkenji@staff.kanazawa-u.ac.jp
First published on 7th August 2019
Abstract
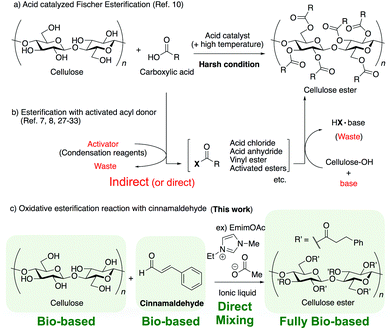
A method of cellulose modification involving simply mixing cellulose with cinnamaldehyde, i.e., “cinnamon flavor”, in an ionic liquid has been developed. An oxidative esterification reaction proceeds between cellulose and cinnamaldehyde to directly provide the highly substituted and formally fully bio-based cellulose ester with perfect atom economy.
Introduction
Current plastics production precursors should be replaced with bio-based materials in order to alleviate the current dependence on petroleum resources.1,2 Polylactic acid (PLA) has a 100% plant constituent ratio, and represents one of the most mass-produced bio-based plastics. However, polymerization from lactide is necessary to obtain high-molecular-weight PLA; this process is indirect and involves at least three steps, lactic acid fermentation from carbohydrates, synthesis of lactide by dehydration condensation of lactic acids, and polymerization of lactide using a metal catalyst (typically, harmful tin octoate); thus, achieving a direct fully bio-based polymer synthesis remains difficult.3,4 Cellulose-based materials, typified by cellulose acetate (CA), have been known as a bio-based polymer for a long time. However, the low solubility of cellulose due to the intra- and inter-molecular hydrogen bonds formed by its three hydroxyl groups complicates its use in materials applications.5,6 In addition, the plant constituent ratio of CA with a degree of substitution (DS) of 2.1 is only about 64%.7 An improved method in terms of plant constituent ratio using cardanol from natural cashews has been reported; however, the plant constituent ratio of the product was only 73%.7,8 Furthermore, these cellulose esters were modified via a classical esterification reaction using alcohols and carboxylic acids, in which it is impossible to avoid the need for an indirect or direct activation process and the resulting generation of waste from the activator (e.g., acid anhydride, condensation agents, etc.) unless the reaction is conducted under harsh conditions using a strong acid (e.g., sulfuric acid)9–12 (Scheme 1a and b). Indirect synthesis involving multiple steps leads to the unnecessary consumption of reagents and energy. Thus, it is necessary to develop an alternative to classical esterification to solve the problem of waste discharge by reducing the amount of processing required and improving the atom economy of the process.13,14In recent years, mild condensation transformation reactions based on oxidative esterification have been developed. These reactions form ester structures between an alcohol and an aldehyde through a N-heterocyclic carbene (NHC) catalyzed type15,16 or acetal type17 oxidation process. Various oxidizers can be used in the oxidation step, including not only external oxidizers such as manganese dioxide and organic agents,18–20 but also internal oxidizers such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the aldehyde compounds.16 For example, it is possible to use cinnamaldehyde, which is a scent component of cinnamon and a natural perfume. Cinnamaldehyde is a non-edible bio-based feedstock and that is present in high concentration in the essential oil obtained from Ceylon cinnamon bark, and has been used industrially as a perfume, pesticide, and rust preventive agent.21 Recently, Zhang and co-workers have reported that cellulose phenylpropionate derivatives, which have a phenylpropionate unit similar to the structure of cinnamaldehyde, show thermoplasticity and are useful as plastics.22 However, compared to classical esterification, oxidative esterification reactions are complicated and expected to result in side reactions due to the use of a powerful oxidizer. Furthermore, oxidative esterification has been considered unsuitable for the modification of poorly soluble cellulose in various solvents, including ionic liquids (ILs),18,23 which are ionic compounds with melting points below 100 °C,24 because they require an excess of the alcohol to prevent the dimerization pathway of the aldehyde.
C bonds in the aldehyde compounds.16 For example, it is possible to use cinnamaldehyde, which is a scent component of cinnamon and a natural perfume. Cinnamaldehyde is a non-edible bio-based feedstock and that is present in high concentration in the essential oil obtained from Ceylon cinnamon bark, and has been used industrially as a perfume, pesticide, and rust preventive agent.21 Recently, Zhang and co-workers have reported that cellulose phenylpropionate derivatives, which have a phenylpropionate unit similar to the structure of cinnamaldehyde, show thermoplasticity and are useful as plastics.22 However, compared to classical esterification, oxidative esterification reactions are complicated and expected to result in side reactions due to the use of a powerful oxidizer. Furthermore, oxidative esterification has been considered unsuitable for the modification of poorly soluble cellulose in various solvents, including ionic liquids (ILs),18,23 which are ionic compounds with melting points below 100 °C,24 because they require an excess of the alcohol to prevent the dimerization pathway of the aldehyde.
In 2002, Roger and co-authors reported that cellulose dissolves in appropriately selected ILs25,26 and the modification of cellulose in ILs under homogenous solvent systems has been investigated (Scheme 1b).27–31 However, these cellulose modifications in ILs were also conducted through classical esterification using an activated acyl donor, producing the corresponding wastes. It is necessary to develop new cellulose modification methods using oxidative esterification in ILs to produce fully bio-based polymers derived from cellulose, which would achieve high sustainability.
Based on the above reports, the authors surmised that the oxidative esterification of cellulose with cinnamaldehyde could be achieved using an IL as both the solvent and an NHC-type catalyst (Scheme 1c). This represents the first attempt to carry out a direct fully bio-based and carbon neutral polymer synthesis with perfect atom economy (formally, it does not cause the loss of even a single hydrogen atom) simply by direct mixing of bio-based cellulose and cinnamaldehyde in ILs without the use of any additional condensation reagents, catalysts, or indirect synthesis process.
Results and discussion
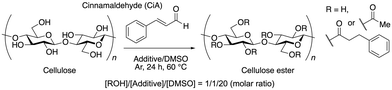
Mixed solvent systems consisting of dimethylsufoxide (DMSO) and 1-ethyl-3-methylimidazolium acetate (EmimOAc) have been reported to dissolve cellulose32 and accelerate some cellulose modification reactions.33 An amount of cinnamaldehyde equimolar with the concentration of the hydroxyl groups of cellulose was added to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (molar ratio) EmimOAc/DMSO solution in which cellulose had been dissolved and heated at 60 °C under an Ar atmosphere for 24 h (Scheme 2). The reaction solution was homogenous throughout the process, indicating that EmimOAc effectively solvated cellulose in this system. After the reaction, the resulting polymer was recovered from the reaction mixture as a fibrous solid by precipitation in MeOH. IR measurements of the obtained cellulose derivatives revealed an absorption band at 1729 cm−1 corresponding to C
20 (molar ratio) EmimOAc/DMSO solution in which cellulose had been dissolved and heated at 60 °C under an Ar atmosphere for 24 h (Scheme 2). The reaction solution was homogenous throughout the process, indicating that EmimOAc effectively solvated cellulose in this system. After the reaction, the resulting polymer was recovered from the reaction mixture as a fibrous solid by precipitation in MeOH. IR measurements of the obtained cellulose derivatives revealed an absorption band at 1729 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and one at 1236 cm−1 corresponding to C–O–C, indicating the presence of an ester group and the successful esterification of the cellulose with cinnamaldehyde (Fig. 1).
O stretching and one at 1236 cm−1 corresponding to C–O–C, indicating the presence of an ester group and the successful esterification of the cellulose with cinnamaldehyde (Fig. 1).
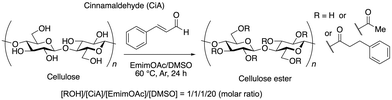 | ||
| Scheme 2 Schematic illustration of the oxidative esterification of cellulose using EmimOAc as both the solvent and the catalyst. | ||
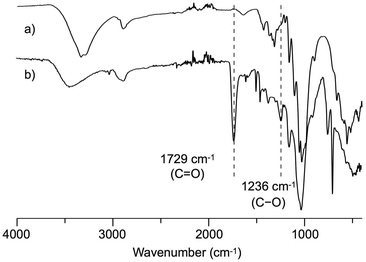 | ||
| Fig. 1 ATR-mode FT-IR spectra of unmodified cellulose (a) and the cellulose esters product shown in Scheme 2(b). | ||
In the 1H NMR spectrum of the obtained product in DMSO-d6 (Fig. 2a), the peaks at 3.0–5.8 ppm correspond to the cellulose backbone, those at 6.7–7.4 ppm to the aromatic ring of cinnamaldehyde, and those at 2.5–3.0 ppm to the two methylene units of the reduced cinnamaldehyde16 structure. Therefore, the desired cellulose phenylpropionate was determined to have been successfully synthesized. The absence of an aldehyde peak at 9.0 ppm indicated that the potential oxy-Michael addition34 between the hydroxyl group and α,β-unsaturated aldehyde did not occur. Furthermore, no peaks in the 5.5–6.5 ppm region corresponding to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group, which could potentially have been introduced by acetalization35,36 between the diol units of cellulose and the aldehyde group of cinnamaldehyde, were observed. In the 13C NMR spectrum, peaks at around 103 ppm derived from anomeric carbon and at 173 ppm derived from the carbonyl carbon were present (Fig. S1†). The 1H, 13C NMR, and IR spectra thus confirmed that the oxidative esterification of cellulose proceeded with high selectively, avoiding the various potential side reactions related to the α,β-unsaturated aldehyde. On the other hand, peaks corresponding to the acetyl group of the anion of EmimOAc were observed at 1.7–2.1 ppm in the 1H NMR spectrum. The integrals of the phenyl and acetyl group peaks indicated that the ratio of the phenylpropionate and acetate groups on cellulose was 7.4
C group, which could potentially have been introduced by acetalization35,36 between the diol units of cellulose and the aldehyde group of cinnamaldehyde, were observed. In the 13C NMR spectrum, peaks at around 103 ppm derived from anomeric carbon and at 173 ppm derived from the carbonyl carbon were present (Fig. S1†). The 1H, 13C NMR, and IR spectra thus confirmed that the oxidative esterification of cellulose proceeded with high selectively, avoiding the various potential side reactions related to the α,β-unsaturated aldehyde. On the other hand, peaks corresponding to the acetyl group of the anion of EmimOAc were observed at 1.7–2.1 ppm in the 1H NMR spectrum. The integrals of the phenyl and acetyl group peaks indicated that the ratio of the phenylpropionate and acetate groups on cellulose was 7.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (molar ratio). The introduction of acetate groups via an undesired side reaction during the esterification of cellulose in EmimOAc using acyl chloride and vinyl esters has previously been reported.28,37,38
1.0 (molar ratio). The introduction of acetate groups via an undesired side reaction during the esterification of cellulose in EmimOAc using acyl chloride and vinyl esters has previously been reported.28,37,38
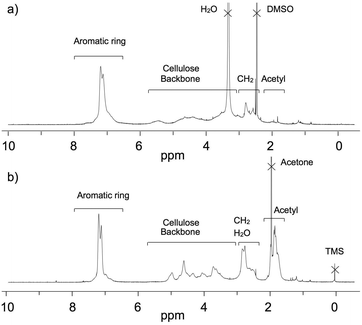 | ||
| Fig. 2 1H NMR spectra of the cellulose ester product shown in Scheme 2 in DMSO-d6 (a) and the cellulose ester product after per-acetylation in acetone-d6 (b) measured at room temperature. | ||
Subsequently, the degree of substitution (DS) of the resulting product was determined. In order to enhance its solubility in acetone-d6 and avoid overlap of sample and solvent peaks, the obtained cellulose ester was completely acetylated with acetic acid and condensation reagent. The integration ratio of the peaks corresponding to the cellulose backbone and the aromatic ring in the 1H NMR spectra in acetone-d6 was determined (Fig. 2b), and the DS values of the desired phenylpropionate group (DSmain) = 1.04 and the undesired acetyl group (DSside) = 0.14 were estimated.
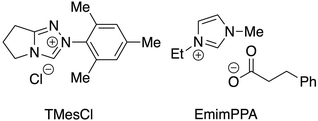
As a control experiment, neat DMSO without EmimOAc was used as the solvent; the cellulose did not dissolve in the resulting solution (Table 1, entry 2). As expected, the band at around 1729 cm−1 which indicated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the ester group and thus successful esterification, was hardly observed in the IR spectrum of the resulting product (Fig. S2†). Based on a previous report of an oxidative esterification reaction using an NHC catalyst and a strong base,16 the oxidative esterification of cellulose was carried out in the presence of a catalytic amount of 2-mesityl-2,5,6,7-tetrahydropyrrolo[2,1-c][1,2,4]triazol-4-ium chloride (TMesCl) (Fig. 3) and diisopropylethylamine (DIPEA) without IL (entry 3). The IR spectrum of the resulting product showed that the reaction barely proceeded because the cellulose was not dissolved (Fig. S2†); this result indicates the necessity for the IL to serve as both the catalyst and solvent.
O stretching of the ester group and thus successful esterification, was hardly observed in the IR spectrum of the resulting product (Fig. S2†). Based on a previous report of an oxidative esterification reaction using an NHC catalyst and a strong base,16 the oxidative esterification of cellulose was carried out in the presence of a catalytic amount of 2-mesityl-2,5,6,7-tetrahydropyrrolo[2,1-c][1,2,4]triazol-4-ium chloride (TMesCl) (Fig. 3) and diisopropylethylamine (DIPEA) without IL (entry 3). The IR spectrum of the resulting product showed that the reaction barely proceeded because the cellulose was not dissolved (Fig. S2†); this result indicates the necessity for the IL to serve as both the catalyst and solvent.
| Entry | Additive | [CiA]/[ROH] | Injection of CiA | DSmain |
|---|---|---|---|---|
a Reaction conditions: [ROH]/[CiA]/[IL]/[DMSO] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (molar ratio); 24 h; 60 °C; Ar atmosphere.
b DSside value derived from the acetate anion.
c Based on the small absorption band at around 1729 cm−1 corresponding to C 20 (molar ratio); 24 h; 60 °C; Ar atmosphere.
b DSside value derived from the acetate anion.
c Based on the small absorption band at around 1729 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in the IR spectrum (Fig. S2).
d [ROH]/[CiA]/[TMesCl]/[DIPEA]/[DMSO] = 1 O stretching in the IR spectrum (Fig. S2).
d [ROH]/[CiA]/[TMesCl]/[DIPEA]/[DMSO] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (molar ratio). 20 (molar ratio).
|
||||
| 1 | EmimOAc | 1 | One shot | 1.04 (0.14)b |
| 2 | None | 1 | One shot | n.d.c |
| 3d | TMesCl | 1 | One shot | n.d.c |
| 4 | EmimOAc | 1 | 50 μL h−1 (6 h) | 1.66 (0.16)b |
| 5 | EmimOAc | 1 | 25 μL h−1 (12 h) | 1.69 (0.17)b |
| 6 | EmimPPA | 1 | 50 μL h−1 (6 h) | 2.04 |
| 7 | EmimPPA | 2 | 50 μL h−1 (12 h) | 2.06 |
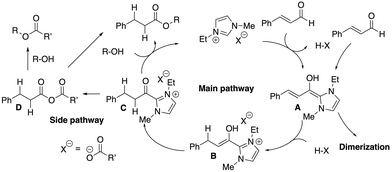
Despite the use of an equimolar amount of cinnamaldehyde, the DS value was only moderate (Table 1, entry 1), leaving room for improvement. Benzoin condensation is known to occur between the Breslow intermediate A generated by imidazolium and the aldehyde,39 and a second molecule of the aldehyde in NHC-catalyzed reaction systems, leading to dimerization of the aldehyde (Fig. 4, right). Furthermore, when an α,β-unsaturated aldehyde is used, the lactonization reaction leading to the dimerization of the α,β-unsaturated aldehyde through the same intermediate A has been reported.16 The cinnamaldehyde-derived Breslow intermediate A likely reacted with another cinnamaldehyde before the acylimidazolium intermediate C could be via the intramolecular redox process, thus decreasing the DS value. To reduce the concentration of cinnamaldehyde in the reaction solution and thus suppress the dimerization pathway, cinnamaldehyde was slowly added over a period of ca. 6 hours at a rate of 50 μL (0.37 mmol) h−1 using a syringe pump, and the DSmain value increased to 1.66 (entry 4). The DS value did not improve significantly (DSmain = 1.69) when a slower rate of 25 μL (0.19 mmol) h−1 was used (entry 5). On the other hand, the acetyl group contamination of the cellulose ester was expected to be caused by the formation of the mixed acid anhydride D and nucleophilic attack of its hydroxyl group on cellulose (Fig. 4, left).38 To avoid the undesired introduction of acetate anions to the cellulose ester, the reaction was carried out using Emim phenylpropionate (EmimPPA) (Fig. 3), in which the anion corresponds to the cellulose phenylpropionate structure. By using the anion to be introduced to cellulose as the IL anion, it was expected that only the symmetric acid anhydride would be generated, and that undesired structures would not be introduced. As a result, the reaction proceeded in a homogenous system, and only the desired phenylpropionate structure was introduced to the resulting product (entry 6), and the DS value increased (DS = 2.04). This result shows that the formally fully bio-based cellulose ester with a formal 100% plant constituent ratio could be developed by simply direct mixing commercially available cellulose and cinnamon flavor in an IL containing solvent system without any additional activation reagents. In this case, the reaction was carried out using 120 mg cellulose and 291 mg cinnamaldehyde ([ROH]/[CiA] = 1/1, molar ratio), for which the theoretical yield of fully substituted cellulose phenylpropionate (DS = 3.0) is 411 mg; 291 mg cellulose phenylpropionate (DS = 2.04) was obtained in 71% isolated yield. This yield was equal to the reaction mass efficiency (RME),40 which is one of the green chemistry metrics. In addition, size exclusion chromatography (SEC) of the cellulose phenylpropionate was performed, and the weight average molecular weight (Mw) was found to be 5.0 × 104 with a molecular weight distribution (Mw/Mn) of 2.5 being fair for natural carbohydrate-based polymer.33 When the amount of cinnamaldehyde was increased, almost no change in the DS value was observed (DS = 2.06) (entry 7).
To compare the difference between this system and the conventional method using an acid chloride, the remaining hydroxyl groups of the resulting cellulose phenylpropionate synthesized by oxidative esterification (DS = 2.04) (Table 1, entry 6) were modified using 3-phenylpropionyl chloride to prepare fully substituted cellulose phenylpropionate (DS > 2.9). Almost no differences were observed between the 1H NMR, 13C NMR and IR spectra of this sample and those of a sample with a similar DS value (DS > 2.9) prepared from cellulose using the conventional method with the corresponding acid chloride (Fig. S3–S5†). Thus, these data indicated that the cellulose ester obtained by oxidative esterification in ILs was chemically almost the same as that obtained by using the classical esterification method.
The solubility of the cellulose phenylpropionate (Table 1, entry 6) was confirmed (Table S1†), and a film of the polymer could be cast from the acetone solution (Fig. 5). The obtained film was transparent, and we were able to visually recognize sentences printed on paper through the film.
 | ||
| Fig. 5 Photographs of the cellulose phenylpropionate film (Table 1, entry 6) cast from an acetone solution (left side). | ||
Conclusions
A method to modify cellulose to obtain a formally fully bio-based plastic derived from plant constituents by simply dissolving commercially available cellulose and cinnamaldehyde, which is a fragrance component of cinnamon, in an ionic liquid (IL) containing solvent system without any additional catalysts has been developed, and shows perfect atom economy. The maximum degree of substitution reached 2.06, and a side reaction in which the anion of the carboxylate type IL was introduced into the cellulose ester was formally avoided by using the newly synthesized IL 1-ethyl-3-methylimidazolium 3-phenylpropionate. By using this IL containing solvent system, aldehydes which have not been successfully used for cellulose esterification can be used, and the feasibility of synthesizing various bio-based plastics via oxidative esterification was confirmed. Mechanistic studies of the oxidative esterification reaction in IL containing solvent systems, including side reactions, optimization of the reaction conditions, the scope of aldehydes, oxidants, and carbohydrates, and the mechanical properties of the obtained polymers are currently in progress.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
S. B. W. K. are thankful for kind support of Indonesian Endowment Fund for Education (LPDP), Ministry of Finance; and Directorate General of Higher Education (DIKTI). This work was supported in part by JSPS KAKENHI Grant No. 18H02253 (Grants-in-Aid for Research (B)) and by JST COI Grant No. JPMJCE1315.Notes and references
- A. Gandini, Macromolecules, 2008, 41, 9491–9504 CrossRef CAS.
- R. Rinaldi, Angew. Chem., Int. Ed., 2014, 53, 8559–8560 CrossRef CAS PubMed.
- H. Tsuji, Macromol. Biosci., 2005, 5, 569–597 CrossRef CAS PubMed.
- T. Iwata, Angew. Chem., Int. Ed., 2015, 54, 3210–3215 CrossRef CAS PubMed.
- K. J. Edgar, C. M. Buchanan, J. S. Debenham, P. A. Rundquist, B. D. Seiler, M. C. Shelton and D. Tindall, Prog. Polym. Sci., 2001, 26, 1605–1688 CrossRef CAS.
- X. S. Fan, Z. W. Liu, J. Lu and Z. T. Liu, Ind. Eng. Chem. Res., 2009, 48, 6212–6215 CrossRef CAS.
- M. Iji, S. Moon and S. Tanaka, Polym. J., 2011, 43, 738–741 CrossRef CAS.
- S. Tanaka, T. Iwata and M. Iji, ACS Sustainable Chem. Eng., 2017, 5, 1485–1493 CrossRef CAS.
- J. Otera, Chem. Rev., 1993, 93, 1449–1470 CrossRef CAS.
- E. Fischer and A. Speier, Ber. Dtsch. Chem. Ges., 1895, 28, 3252–3258 CrossRef CAS.
- T. vom Stein, P. Grande, F. Sibilla, U. Commandeur, R. Fischer, W. Leitner and P. Domínguez de María, Green Chem., 2010, 12, 1844–1849 RSC.
- S. Spinella, A. Maiorana, Q. Qian, N. J. Dawson, V. Hepworth, S. A. McCallum, M. Ganesh, K. D. Singer and R. A. Gross, ACS Sustainable Chem. Eng., 2016, 4, 1538–1550 CrossRef CAS.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259–281 CrossRef CAS.
- S. J. Ryan, L. Candish and D. W. Lupton, Chem. Soc. Rev., 2013, 42, 4906–4917 RSC.
- S. S. Sohn and J. W. Bode, Org. Lett., 2005, 7, 3873–3876 CrossRef CAS PubMed.
- R. Gopinath and B. K. Patel, Org. Lett., 2000, 2, 577–579 CrossRef CAS PubMed.
- I. Chiarotto, M. Feroci, G. Sotgiu and A. Inesi, Tetrahedron, 2013, 69, 8088–8095 CrossRef CAS.
- C. E. I. Knappke, A. Imami and A. Jacobi von Wangelin, ChemCatChem, 2012, 4, 937–941 CrossRef CAS.
- R. C. Samanta and A. Studer, Org. Chem. Front., 2014, 1, 936–939 RSC.
- G. Singh, S. Maurya, M. P. deLampasona and C. A. N. Catalan, Food Chem. Toxicol., 2007, 45, 1650–1661 CrossRef CAS PubMed.
- Z. Y. Chen, J. M. Zhang, P. Xiao, W. G. Tian and J. Zhang, ACS Sustainable Chem. Eng., 2018, 6, 4931–4939 CrossRef CAS.
- Y. Y. Yu, L. Hua, W. W. Zhu, Y. Shi, T. Cao, Y. X. Qiao and Z. S. Hou, Synth. Commun., 2013, 43, 1287–1298 CrossRef CAS.
- Z. Radai, N. Z. Kiss and G. Keglevich, Curr. Org. Chem., 2018, 22, 533–556 CrossRef CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- M. J. Chen, R. M. Li, X. Q. Zhang, J. Feng, J. Feng, C. F. Liu and Q. S. Shi, ACS Sustainable Chem. Eng., 2017, 5, 360–366 CrossRef CAS.
- L. P. Hinner, J. L. Wissner, A. Beurer, B. A. Nebel and B. Hauer, Green Chem., 2016, 18, 6099–6107 RSC.
- R. Kakuchi, M. Yamaguchi, T. Endo, Y. Shibata, K. Ninomiya, T. Ikai, K. Maeda and K. Takahashi, RSC Adv., 2015, 5, 72071–72074 RSC.
- T. Takeshita, A. Kitagawa, F. Yokosu, R. Matsumoto, T. Nokami and T. Itoh, Aust. J. Chem., 2018, 72, 61–69 CrossRef.
- S. Barthel and T. Heinze, Green Chem., 2006, 8, 301–306 RSC.
- M. Gericke, T. Liebert, O. A. El Seoud and T. Heinze, Macromol. Mater. Eng., 2011, 296, 483–493 CrossRef CAS.
- R. Kakuchi, R. Ito, S. Nomura, H. Abroshan, K. Ninomiya, T. Ikai, K. Maeda, H. J. Kim and K. Takahashi, RSC Adv., 2017, 7, 9423–9430 RSC.
- T. Kano, Y. Tanaka and K. Maruoka, Tetrahedron, 2007, 63, 8658–8664 CrossRef CAS.
- N. M. Leonard, M. C. Oswald, D. A. Freiberg, B. A. Nattier, R. C. Smith and R. S. Mohan, J. Org. Chem., 2002, 67, 5202–5207 CrossRef CAS PubMed.
- Y. Kuroiwa, M. Sekine, S. Tomono, D. Takahashi and K. Toshima, Tetrahedron Lett., 2010, 51, 6294–6297 CrossRef CAS.
- S. Kohler, T. Liebert, M. Schobitz, J. Schaller, F. Meister, W. Gunther and T. Heinze, Macromol. Rapid Commun., 2007, 28, 2311–2317 CrossRef.
- D. Hirose, S. B. W. Kusuma, S. Nomura, M. Yamaguchi, Y. Yasaka, R. Kakuchi and K. Takahashi, RSC Adv., 2019, 9, 4048–4053 RSC.
- D. J. Liu, Y. T. Zhang and E. Y. X. Chen, Green Chem., 2012, 14, 2738–2746 RSC.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of ionic liquids and cellulose esters. See DOI: 10.1039/c9gc01333d |
| This journal is © The Royal Society of Chemistry 2019 |