The periodic table of the elements of green and sustainable chemistry
Abstract
Achieving a sustainable future will only be possible through the intersection of the best science and technology in combination with the societal, economic, policy, cultural, moral, and ethical ecosystem. Green chemistry and green engineering provide the scientific and technological foundation of the elements of green and sustainable chemistry while the other elements, relating to humanitarian aims, enabling system conditions, and noble goals, provide the imperative context. This alternative periodic table, strives to outline the range of aspects and tools that are available and needed to accomplish the daunting and necessary tasks of moving toward a sustainable tomorrow.
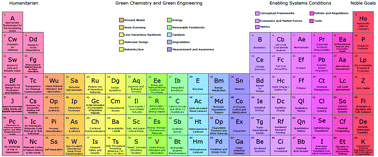
- This article is part of the themed collections: International Symposium on Green Chemistry 2019 and 2019 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...